Raloxifene inhibits the overexpression of TGF-β1 in cartilage and regulates the metabolism of subchondral bone in rats with osteoporotic osteoarthritis
DOI:
https://doi.org/10.17305/bjbms.2020.5142Keywords:
Osteoporosis, osteoarthritis, raloxifene, TGF-β1, cartilage, subchondral boneAbstract
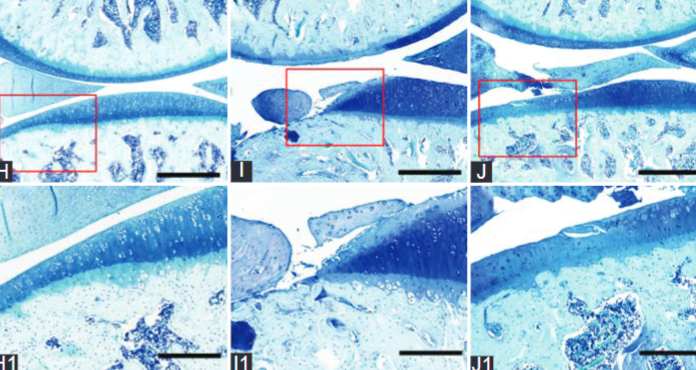
Overexpression of transforming growth factor-beta 1 (TGF-β1) and subchondral bone remodelling play key roles in osteoarthritis (OA). Raloxifene (RAL) reduces the serum level of TGF-β1 in postmenopausal women. However, the effect of RAL on TGF-β1 expression in articular cartilage is still unclear. Therefore, we aimed to investigate the protective effect of RAL on osteoporotic osteoarthritis via affecting TGF-β1 expression in cartilage and the metabolism of subchondral bone. Osteoporotic osteoarthritis was induced by a combination of anterior cruciate transection (ACLT) and ovariectomy (OVX). Rats were divided into five groups (n = 12): The sham group, the ACLT group, the OVX group, the ACLT + OVX group, and the RAL group (ACLT + OVX + RAL, 6.25 mg/kg/day for 12 weeks). Assessment was performed by histomorphology, microcomputed tomography (micro-CT) scan, immunohistochemistry, and tartrate-resistant acid phosphatase (TRAP) staining. We found that severe cartilage degeneration was shown in the ACLT + OVX group. The histomorphological scores, the levels of TGF-β1, and its related catabolic enzymes and osteoclasts numbers in the ACLT + OVX group were higher than those in other groups (p < 0.05). Furthermore, structure model index (SMI) and trabecular spacing (Tb.Sp) were decreased (p < 0.05), while bone mineral density (BMD), bone volume fraction (BV/TV), and trabecular number (Tb.N) were increased by RAL compared with the ACLT + OVX group (p < 0.05). Our findings demonstrated that RAL in clinical doses retards the development of osteoporotic osteoarthritis by inhibiting the overexpression of TGF-β1 in cartilage and regulating the metabolism of subchondral bone. These results provide support for RAL in the expansion of clinical indication for prevention and treatment in postmenopausal osteoarthritis.
Citations
Downloads
Downloads
Additional Files
Published
License
Copyright (c) 2020 Shao-Hua Ping, Fa-Ming Tian, Hao Liu , Qi Sun, Li-Tao Shao, Qiang-Qiang Lian, Liu Zhang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2020-11-16
Published 2021-06-01









