Efficacy and safety of PEG-rhG-CSF in preventing chemoradiotherapy-induced neutropenia in patients with locally advanced cervical cancer
DOI:
https://doi.org/10.17305/bjbms.2022.7859Keywords:
Cervical cancer, PEG-rhG-CSF, concurrent chemoradiotherapy, radical chemoradiotherapy, neutropeniaAbstract
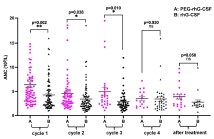
The standard of care for locally advanced cervical cancer is concurrent chemoradiotherapy, which is associated with significant toxicity, especially hematologic toxicity. To evaluate the efficacy and safety of pegylated recombinant human granulocyte colony-stimulating factor (PEG-rhG-CSF) in preventing neutropenia during radical chemoradiotherapy for cervical cancer, 40 patients receiving prophylaxis from February 2018 to July 2019 were randomly divided into two arms in a 1:1 ratio. Patients in the study arm (N = 21) received PEG-rhG-CSF, while patients in the control arm (N = 19) received short-acting rhG-CSF. The primary endpoint was the incidence of grade 3–4 neutropenia, and the secondary endpoints were the incidence of febrile neutropenia, chemotherapy delay, and radiotherapy interruption. In addition, dynamic changes in absolute neutrophil count during radical chemoradiotherapy and adverse events were compared between the two groups. There were 0 and 4 cycles of grade 3–4 neutropenia in the PEG-rhG-CSF and rhG-CSF groups, respectively. The incidence of neutropenia of all grades was lower in patients on PEG-rhG-CSF than on rhG-CSF [24.05% (19/79) vs. 56.94% (41/72); p < 0.001]. No patient developed neutropenic fever. The lowest values of neutropenia during concurrent chemoradiotherapy cycles were 2.73 ± 1.02 and 1.91 ± 0.79 × 10 9/ml in the PEG-rhG-CSF and rhG-CSF groups, respectively (p < 0.001). In the PEG-rhG-CSF and rhG-CSF groups, 0 and 8 (11.11%) cycles of chemotherapy were delayed due to neutropenia, respectively (p = 0.01). There was no delay of radiotherapy by more than one week in either group. Prophylactic use of PEG-rhG-CSF during chemoradiotherapy for cervical cancer can effectively prevent neutropenia and associated adverse events. PEG-rhG-CSF may be an effective strategy to provide uninterrupted radical chemoradiotherapy for cervical cancer.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Weiwei Li, Mohan Dong, Shigao Huang, Liu Shi , Hua Yang, Ying Zhang, Jie Gong, Mei Shi, Lichun Wei, Lina Zhao

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-09-04
Published 2023-03-16









