Changes of placental three-dimensional power Doppler ultrasonography in third trimester among hypertensive disorders complicating pregnancy
DOI:
https://doi.org/10.17305/bb.2023.9085Keywords:
3D power Doppler ultrasonography, hypertensive disorders complicating pregnancy, flow index, vascularization index, vascularization flow indexAbstract
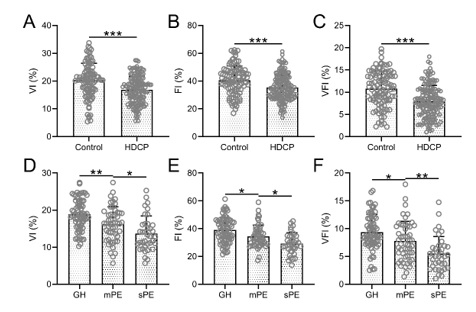
Hypertensive disorders complicating pregnancy (HDCP) represent a systemic condition specific to pregnant women. Three-dimensional (3D) power Doppler ultrasonography is a technique that utilizes erythrocyte density, scattered intensity, or energy distribution in the bloodstream for imaging purposes. This study aimed to compare the changes in 3D power Doppler ultrasonography parameters in late pregnancy between patients with HDCP and those without HDCP, and to evaluate the predictive value of these parameters for pregnancy outcomes in patients with HDCP. The study included 160 pregnant women diagnosed with HDCP and 100 pregnant women without HDCP, who served as the control group. 3D power Doppler ultrasonography was performed, and the values of the vascularization index (VI), flow index (FI), and vascularization flow index (VFI) were measured. In the HDCP group, the VI, FI, and VFI were all lower than those observed in patients without HDCP. In HDCP patients with positive outcomes, these three parameters were higher than those recorded in patients with negative outcomes. The area under the predicted curve (AUC) for VI, FI, VFI, and the combination of these three parameters were 0.69, 0.63, 0.66, and 0.75, respectively. The parameters of 3D power Doppler ultrasonography can reflect the perfusion status of the placenta and predict the outcome of pregnancy in patients with HDCP. By monitoring these relevant hemodynamic parameters, valuable information can be provided for the clinical diagnosis, objective evaluation, and treatment of HDCP.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Yingying Liu, Ximei Gao , Dandan Shi , Yong Wang , Xinying Qi , Na Wang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-04-28
Published 2023-09-04









