Effects of the CB1 receptor antagonists AM6545 and AM4113 on metabolic syndrome-induced prostatic hyperplasia in rats
DOI:
https://doi.org/10.17305/bb.2023.9173Keywords:
AM6545, AM4113, prostate, cannabinoid antagonist, metabolic syndrome (MetS)Abstract
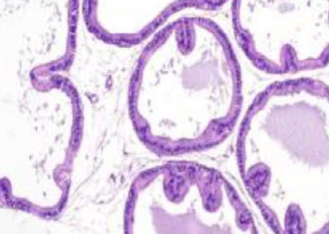
Metabolic syndrome (MetS) is a combination of metabolic disorders that can predispose individuals to benign prostatic hyperplasia (BPH). The inhibition of the cannabinoid 1 (CB1) receptor has been used to treat metabolic disorders in animal models. This study reports the use of a peripherally restricted CB1 antagonist (AM6545) and a neutral CB1 antagonist (AM4113) to improve MetS-related BPH in rats. Animals were divided into three control groups to receive either a normal rodent diet, AM6545, or AM4113. MetS was induced in the fourth, fifth, and sixth groups using a concentrated fructose solution and high-salt diet delivered as food pellets for eight weeks. The fifth and sixth groups were further given AM6545 or AM4113 for additional four weeks. Body and prostate weights were measured and prostate sections were stained with hematoxylin eosin. Cyclin D1, markers of oxidative stress and inflammation, and levels of the endocannabinoids were recorded. BPH in rats with MetS was confirmed through increased prostate weight and index, as well as histopathology. Treatment with either AM6545 or AM4113 significantly decreased prostate weight, improved prostate histology, and reduced cyclin D1 expression compared with the MetS group. Groups treated with CB1 antagonists experienced reduced lipid peroxidation, recovered glutathione depletion, restored catalase activity, and had lower inflammatory markers interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α). MetS rats treated with either AM6545 or AM4113 showed reduced concentrations of anandamide (AEA) and 2-arachidonoylglycerol (2-AG) in the prostate compared with the MetS group. In conclusion, the CB1 antagonists AM6545 and AM4113 protect against MetS-induced BPH through their anti-proliferative, antioxidant, and anti-inflammatory effects.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Basma G. Eid, Thikryat Neamatallah, Lenah S. Binmahfouz, Amina M. Bagher, Abdulmohsin J. Alamoudi, Hibah Mubarak Aldawsari, Abeer Hanafy, Atif Hasan, Hany M. El-Bassossy, Ashraf B. Abdel-Naim, Kiran Vemuri, Alexandros Makriyannis

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-05-16
Published 2023-11-03









