Silencing METTL14 alleviates liver injury in non-alcoholic fatty liver disease by regulating mitochondrial homeostasis
DOI:
https://doi.org/10.17305/bb.2023.9698Keywords:
Non-alcoholic fatty liver disease (NAFLD), methyltransferase-like 14 (METTL14), mitochondrial homeostasis, imbalance, N6-methyladenosine (m6A) modification, miR-34a-5pAbstract
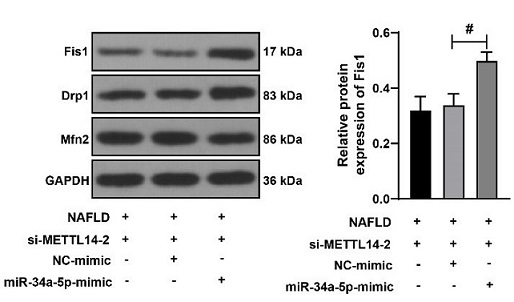
Mitochondrial dysfunction is an important pathogenic factor in non-alcoholic fatty liver disease (NAFLD). Methyltransferase-like 14 (METTL14) has been implicated in mitochondrial fission processes. This research aimed to investigate the mechanism of METTL14 in the mitochondrial function of NAFLD. We first established NAFLD mouse models and cell models, recording body and liver weights and examining pathological changes in liver tissues. Subsequently, serum levels of liver function indices (aspartate aminotransferase [AST], alanine aminotransferase [ALT], total cholesterol [TC], and triglycerides [TG]), inflammatory markers (tumor necrosis factor-alpha [TNF-α], interleukin [IL]-6, and IL-1β), and mitochondrial dysfunction indicators (fission 1 protein [Fis1], dynamin-related protein 1 [Drp1], mitofusin 2 [Mfn2], SID1 transmembrane family member 2 [SIDT2], and mitochondrial membrane potential [MMP]) in the liver and cells were evaluated. The N6-methyladenosine (m6A) modification level of primary microRNA (pri-miRNA) and m6A enrichment on pri-miR-34a were quantified. Co-immunoprecipitation and dual-luciferase reporter gene assays were utilized to validate gene interactions. Our findings revealed highly elevated METTL14 expression in NAFLD mouse and cell models. Silencing METTL14 reduced weight gain and mitigated adverse liver function indices, inflammation, hepatic steatosis, and structural damage in NAFLD mice. It also led to a decrease in Fis1/Drp1 levels and an increase in MMP/Mfn2 in the liver and cells. Moreover, METTL14 increased the m6A level, promoting the binding of DiGeorge syndrome critical region 8 (DGCR8) to pri-miR-34a, which enhanced miR-34a-5p expression. Databases and dual-luciferase reporter gene assays indicated that miR-34a-5p could suppress SIDT2 expression. The overexpression of miR-34a-5p or inhibition of SIDT2 expression negated the alleviative effects of METTL14 silencing on mitochondrial homeostasis imbalance. In conclusion, METTL14, through m6A modification, modulates the miR-34a-5p/SIDT2 axis, impairing mitochondrial homeostasis in NAFLD.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2023 Wei Wang, Jun Yan, Long Han, Zi-Lin Zou, Ai-Lei Xu

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-10-11
Published 2024-05-02









