PARP9 affects myocardial function through TGF-β/Smad axis and pirfenidone
DOI:
https://doi.org/10.17305/bb.2024.10246Keywords:
Cardiac arrhythmias, poly (ADP-ribose) polymerase 9 (PARP9), pirfenidone, TGF-β/Smad signaling pathway, myocardial fibrosisAbstract
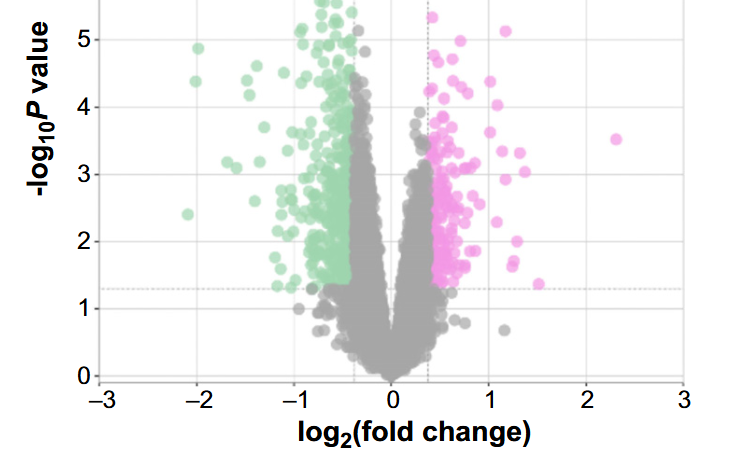
Cardiac arrhythmias are often linked to the overactivity of cardiac fibroblasts (CFs). Investigating the impact of poly (ADP-ribose) polymerase 9 (PARP9) on Angiotensin II (Ang II)-induced fibroblast activation and the therapeutic effects of pirfenidone (PFD) offers valuable insights into cardiac arrhythmias. This study utilized weighted gene co-expression network analysis (WGCNA), differential gene expression (DEG) analysis, protein–protein interaction (PPI), and receiver operating characteristic (ROC) analysis on the GSE42955 dataset to identify the hub gene with a significant diagnostic value. The ImmuCellAI tool revealed an association between PARP9 and immune cell infiltration. Our in vitro assessments focused on the influence of PFD on myofibroblast differentiation, transforming growth factor-beta (TGF-β) expression, and Ang II-induced proliferation and migration in CFs. Additionally, we explored the impact on fibrosis markers and the TGF-β/Smad signaling pathway in the context of PARP9 overexpression. Analysis of the GSE42955 dataset revealed PARP9 as a central gene with high clinical diagnostic value, linked to seven types of immune cells. The in vitro studies demonstrated that PFD significantly mitigates Ang II-induced CF proliferation, migration, and fibrosis. It also reduces Ang II-induced PARP9 expression and decreases fibrosis markers, including TGF-β, collagen I, collagen III, and α-SMA. Notably, PARP9 overexpression can partially counteract PFD’s inhibitory effects on CFs and modify the expression of fibronectin, CTGF, α-SMA, collagen I, collagen III, MMP2, MMP9, TGF-β, and p-Smad2/3 in the TGF-β/Smad signaling pathway. In summary, our findings suggest that PFD effectively counteracts the adverse effects of Ang II-induced CF proliferation and fibrosis, and modulates the TGF-β/Smad signaling pathway and PARP9 expression. This identifies a potential therapeutic approach for managing myocardial fibrosis.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Nannan Chen, Lianzhi Zhang, Zhang Zhong, Wenjia Zhang, Qunlin Gong, Nan Xu, Yimeng Zhou, Jiahong Wang, Pengxiang Zheng

This work is licensed under a Creative Commons Attribution 4.0 International License.









