Immune-related gene expression in severe periodontitis assessed by NanoString technology: A preliminary study
DOI:
https://doi.org/10.17305/bb.2025.13313Keywords:
Severe periodontitis, inflammation, gene expression, differentially expressed genes, DEGs, NanoString technologyAbstract
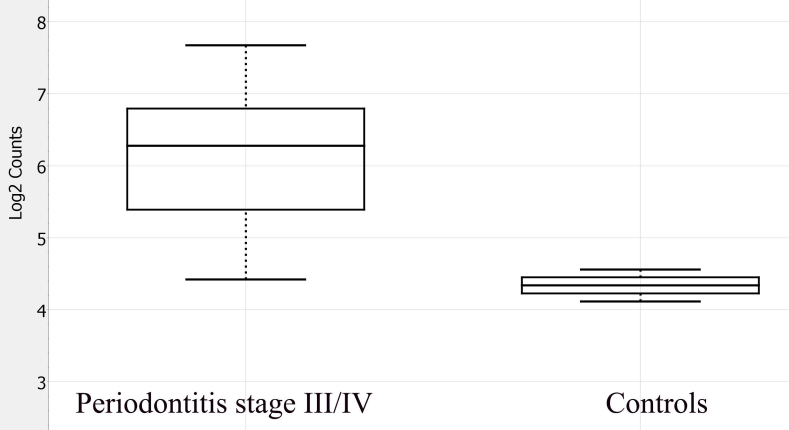
Periodontitis is an inflammatory disease characterized by the destruction of the periodontal attachment apparatus, which includes alveolar bone, periodontal ligament, and cementum. This destruction is driven by a dysregulated host immune response to pathogenic subgingival biofilm. The present preliminary study aimed to evaluate immune-related gene expression patterns in patients with stage III/IV periodontitis utilizing the NanoString nCounter® platform. Unstimulated saliva samples were collected from twelve individuals: ten with severe periodontitis (stage III/IV) and two periodontally healthy controls. Total RNA was isolated and analyzed using the nCounter® Human Inflammation Panel, which profiles 249 inflammation-associated human genes. Data normalization and differential expression analysis were performed with nSolver™ software. Following quality control, genes with low expression (mean normalized counts < 20) were excluded, resulting in 89 genes available for comparison. Among these, 26 genes (29.2%) met a predefined effect-size threshold (|log2FC| ≥ 1), comprising 23 upregulated and 3 downregulated transcripts in the periodontitis group. Notably, the upregulated genes HLA-DRB1 (p = 0.003; FDR = 0.267) and CCR1 (p = 0.007; FDR = 0.312) exhibited relatively large log2 fold changes and the lowest unadjusted p-values; however, neither retained significance after FDR correction. These findings underscore the feasibility of salivary gene expression profiling as a method for identifying molecular markers associated with disease severity. Given their roles in immune activation and leukocyte recruitment, HLA-DRB1 and CCR1 emerge as potential biomarker candidates for detection, risk stratification, and therapeutic monitoring in periodontitis, necessitating validation in larger, well-characterized cohorts.
Citations
Downloads
References
Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020; 83(1):26-39.
https://doi.org/10.1111/prd.12297
Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018; 149(7):576-588.e6.
https://doi.org/10.1016/j.adaj.2018.04.023
Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe tooth loss: a systematic review and meta-analysis. J Dent Res. 2014; 93(7 Suppl):20S-28S.
https://doi.org/10.1177/0022034514537828
Nibali L, Parkar M, Brett P, Knight J, Tonetti MS, Griffiths GS. NADPH oxidase (CYBA) and FcgammaR polymorphisms as risk factors for aggressive periodontitis: a case-control association study. J Clin Periodontol. 2006; 33(8):529-539.
https://doi.org/10.1111/j.1600-051X.2006.00952.x
Shaddox LM, Gonçalves PF, Vovk A, Allin N, Huang H, Hou W, et al. LPS-induced inflammatory response after therapy of aggressive periodontitis. J Dent Res. 2013; 92(8):702-708.
https://doi.org/10.1177/0022034513495242
Schäfer AS, Jepsen S, Loos BG. Periodontal genetics: a decade of genetic association studies mandates better study designs. J Clin Periodontol. 2011; 38(2):103-107.
https://doi.org/10.1111/j.1600-051X.2010.01653.x
Loos BG, Chin DPM. What is the extent of the role played by genetic factors in the aetiology of periodontitis? Perio Insight. 2017; 4:1-8.
Ban Q, Ling X, Ding H, Meng J, Li Y, Zhang J, et al. Screening of periodontitis-related genes and immune cells based on an integrated bioinformatics analysis. Ann Transl Med. 2022; 10(10):558.
https://doi.org/10.21037/atm-22-1592
Cárdenas AM, Ardila LJ, Vernal R, Melgar-Rodríguez S, Hernández HG. Biomarkers of periodontitis and its differential DNA methylation and gene expression in immune cells: a systematic review. Int J Mol Sci. 2022; 23(19):12042.
https://doi.org/10.3390/ijms231912042
Li H, Du W, Ye X, Luo X, Duan X. Genetic analysis of potential markers and therapeutic targets for immunity in periodontitis. Front Dent Med. 2024; 5:1480346.
https://doi.org/10.3389/fdmed.2024.1480346
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018; 89(Suppl 1):S159-S172.
https://doi.org/10.1002/JPER.18-0006
Menezes CFS, Lage LM, Santos LGS, Nascimento GC, Magalhães M. HLA polymorphisms linked to the severity and extent of periodontitis in patients with type 1 diabetes from a Brazilian mixed population. Int J Environ Res Public Health. 2025; 22(4):512.
https://doi.org/10.3390/ijerph22040512
Roshna T, Thomas R, Nandakumar K, Banerjee M. A case-control study on the association of human leukocyte antigen-A9 and -B15 alleles with generalized aggressive periodontitis in an Indian population. J Periodontol. 2006; 77(12):1954-1963.
https://doi.org/10.1902/jop.2006.040411
Repeke CE, Ferreira SB, Claudino M, Silveira EM, de Assis GF, Avila-Campos MJ, et al. Evidences of the cooperative role of the chemokines CCL3, CCL4 and CCL5 and its receptors CCR1+ and CCR5+ in RANKL+ cell migration throughout experimental periodontitis in mice. Bone. 2010; 46(4):1122-1130.
https://doi.org/10.1016/j.bone.2009.12.030
Germano DB, Silveira ALPA, Kim YJ, do Amaral JB, Shio MT, da Silva Nali LH, et al. Expression of monocyte chemokine receptors in diabetes after non-surgical periodontal treatment: a pilot study. Cytokine. 2024; 178:156579.
https://doi.org/10.1016/j.cyto.2024.156579
Szczepaniak P, Mikolajczyk TP, Czesnikiewicz-Guzik M, Guzik TJ. Periodontitis as an inflammatory trigger in hypertension: from basic immunology to clinical implications. Kardiol Pol. 2021; 79(11):1206-1214.
https://doi.org/10.33963/KP.a2021.0161
Fageeh HN, Fageeh HI, Khan SS, Maganur PC, Vyas N, Patil VR, et al. Gingival crevicular fluid infiltrating CD14+ monocytes promote inflammation in periodontitis. Saudi J Biol Sci. 2021; 28(5):3069-3075.
https://doi.org/10.1016/j.sjbs.2021.02.049
de Vries TJ, Andreotta S, Loos BG, Nicu EA. Genes critical for developing periodontitis: lessons from mouse models. Front Immunol. 2017; 8:1395.
https://doi.org/10.3389/fimmu.2017.01395
Chen Z, Lang G, Xu X, Liang X, Han Y, Han Y. The role of NF-kappaB in the inflammatory processes related to dental caries, pulpitis, apical periodontitis, and periodontitis - a narrative review. PeerJ. 2024; 12:e17953.
https://doi.org/10.7717/peerj.17953
Nilsson BO. Mechanisms involved in regulation of periodontal ligament cell production of pro-inflammatory cytokines: implications in periodontitis. J Periodont Res. 2021; 56(2):249-255.
https://doi.org/10.1111/jre.12823
Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and chemokines in periodontitis. Eur J Dent. 2020; 14(3):483-495.
https://doi.org/10.1055/s-0040-1712718
Huang Y, Yang J, Zhang Y, Kuang S, Shen Z, Qin W. Blocking CXCR1/2 attenuates experimental periodontitis by suppressing neutrophils recruitment. Int Immunopharmacol. 2024; 128:111465.
https://doi.org/10.1016/j.intimp.2023.111465
Hajishengallis G, Moutsopoulos NM. Etiology of leukocyte adhesion deficiency-associated periodontitis revisited: not a raging infection but a raging inflammatory response. Expert Rev Clin Immunol. 2014; 10(8):973-975.
https://doi.org/10.1586/1744666X.2014.929944
Luchian I, Goriuc A, Sandu D, Covasa M. The role of matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Int J Mol Sci. 2022; 23(3):1806.
https://doi.org/10.3390/ijms23031806
Radzki D, Negri A, Kusiak A, Obuchowski M. Matrix metalloproteinases in the periodontium - vital in tissue turnover and unfortunate in periodontitis. Int J Mol Sci. 2024; 25(5):2763.
https://doi.org/10.3390/ijms25052763
Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. 2017; 8:910.
https://doi.org/10.3389/fphys.2017.00910
Patil RT, Dhadse PV, Salian SS, Punse SD. Role of oxidative stress in periodontal diseases. Cureus. 2024; 16(5):e60779.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2025 Dragomira Nikolova, Velitchka Dosseva-Panova, Dimitar Dimitrov, Savina Hadjidekova, Ivanka Dimova

This work is licensed under a Creative Commons Attribution 4.0 International License.









