Comparing del Nido and St. Thomas II cardioplegia in a rat ischemia–reperfusion model: Histopathology, mitochondria, and TEM analysis
DOI:
https://doi.org/10.17305/bb.2025.13394Keywords:
Cardioplegia, myocardial ischemia, reperfusion injury, del Nido, St. Thomas II, transmission electron microscopy, histopathology, rat modelAbstract
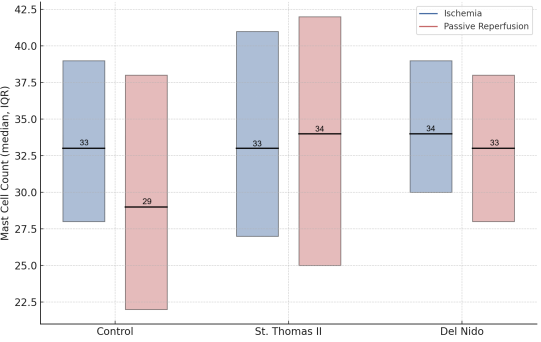
Myocardial ischemia–reperfusion (IR) injury remains a major challenge in cardiac surgery, and comparative histological and ultrastructural data on cardioplegia solutions are limited. This study compared the myocardial protective effects of St. Thomas II and del Nido cardioplegia in a controlled rat IR model, focusing on inflammation, mast cell dynamics, and subcellular preservation. Twenty-four Wistar Albino rats were randomized to Control, St. Thomas II, or del Nido groups. After 90 minutes of ischemia and 30 minutes of passive reperfusion, myocardial tissue was analyzed by hematoxylin–eosin, toluidine blue, and transmission electron microscopy. Outcomes included mast cell counts, leukocyte infiltration, karyolysis, and ultrastructural measures (Flameng score, crista density, basement membrane thickness). Both cardioplegia groups preserved myocardial morphology and attenuated inflammatory changes versus control. Light microscopy revealed a consistent mast cell density and reduced karyolysis in hearts treated with cardioplegia, with no significant differences observed between St. Thomas II and del Nido solutions. Conversely, transmission electron microscopy (TEM), the primary endpoint of this study, demonstrated enhanced mitochondrial and endothelial preservation in the del Nido group, as evidenced by significantly lower Flameng scores and increased crista density compared to both St. Thomas II and control groups (p < 0.05). In conclusion, both solutions reduced early IR-related injury, but del Nido provided a significant ultrastructural advantage on TEM despite similar routine light-microscopic findings.
Citations
Downloads
References
Squiccimarro E, Nicolini F, Agostinelli A, Gherli T. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif Organs. 2022;46(4):568–77.
https://doi.org/10.1111/aor.14171
Jucá FG, da Silva Almeida T, de Lima Gurgel R, et al. Difference between cardiopulmonary bypass time and aortic cross-clamping time as a predictor of complications after coronary artery bypass grafting. Braz J Cardiovasc Surg. 2024;39(2):e20230104.
https://doi.org/10.21470/1678-9741-2023-0104
Chen M, Li X, Mu G. Myocardial protective and anti-inflammatory effects of dexmedetomidine in patients undergoing cardiovascular surgery with cardiopulmonary bypass: a systematic review and meta-analysis. J Anesth. 2022;36(1):5–16.
https://doi.org/10.1007/s00540-021-02982-0
Abbasciano RG, Barlow CW, Ng A, et al. Role of hypothermia in adult cardiac surgery patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2022;36(7):1883–90.
https://doi.org/10.1053/j.jvca.2022.01.026
Francica A, Santarpino G, Fischlein T. Cardioplegia between evolution and revolution: from depolarized to polarized cardiac arrest in adult cardiac surgery. J Clin Med. 2021;10(19):4485.
https://doi.org/10.3390/jcm10194485
Tan J, Zhang H, Li Y, et al. Comparative effects of different types of cardioplegia in cardiac surgery: a network meta-analysis. Front Cardiovasc Med. 2022;9:996744.
https://doi.org/10.3389/fcvm.2022.996744
Tang S, Zhao J, Liu J, et al. The interval time for the St. Thomas cardioplegia solution in mitral valve surgeries. BMC Cardiovasc Disord. 2024;24(1):665.
https://doi.org/10.1186/s12872-024-04328-6
Awad AK, Mahmoud A, Sabry M, et al. Which is better for pediatric and adult cardiac surgery: del Nido or St. Thomas cardioplegia? A systematic review and meta-analysis. Indian J Thorac Cardiovasc Surg. 2023;39(6):588–600.
https://doi.org/10.1007/s12055-023-01553-0
Zhai K, Li J, Wang Z, et al. Del Nido cardioplegia for myocardial protection in adult cardiac surgery: a systematic review and update meta-analysis. Perfusion. 2023;38(1):6–17.
https://doi.org/10.1177/02676591211031095
Moktan Lama PB, Yadav A, Sharma B, et al. Del Nido cardioplegia in coronary artery bypass grafting surgery: a safe, efficacious and economic alternative to St. Thomas solution; an experience from a developing nation. Perfusion. 2021;36(5):470–5.
https://doi.org/10.1177/0267659121991033
Rizvi MFA, Qureshi F, Ahmad A, et al. Prospective randomized study comparing outcome of myocardial protection with del Nido cardioplegia versus Saint Thomas cardioplegia in adult cardiac surgical patients. Pak J Med Sci. 2022;38(3 Pt 1):699–704.
https://doi.org/10.12669/pjms.38.3.4730
Xiong W, Wang L, Chen Q, et al. Dexmedetomidine preconditioning mitigates myocardial ischemia/reperfusion injury via inhibition of mast cell degranulation. Biomed Pharmacother. 2021;141:111853.
https://doi.org/10.1016/j.biopha.2021.111853
He Z, Liu Y, Ye Y, et al. Activation mechanisms and multifaceted effects of mast cells in ischemia reperfusion injury. Exp Cell Res. 2019;376(2):227–35.
https://doi.org/10.1016/j.yexcr.2019.01.022
Collins HE, Neuman JC, Lahm T. Mitochondrial morphology and mitophagy in heart diseases: qualitative and quantitative analyses using transmission electron microscopy. Front Aging. 2021;2:670267.
https://doi.org/10.3389/fragi.2021.670267
Jung JC, Park JH, Lee SH, et al. Serial ultrastructural evaluation of myocardial ischemic injury after infusion of del Nido cardioplegia in the human heart. J Thorac Cardiovasc Surg. 2022;164(2):528–35.
https://doi.org/10.1016/j.jtcvs.2020.08.083
Bushi G, Reddy K, Sharma M. Optimizing cardioplegia: reducing metabolic stress in coronary artery bypass surgery. Int J Surg Open. 2024;62(6):850–1.
https://doi.org/10.1097/IO9.0000000000000231
Brown AJ, Chambers DJ. Physiology and cardioplegia: safety in operating. Surgery (Oxford). 2021;39(3):126–31.
https://doi.org/10.1016/j.mpsur.2021.01.008
Zhang X, Du Y, Wang A. Protective efficacy on adult ischemic myocardium under bypass: del Nido vs. St. Thomas blood cardioplegia. Ann Thorac Cardiovasc Surg. 2023;29(3):125–32.
https://doi.org/10.5761/atcs.oa.22-00144
Nowicki R, Śliwka J, Janowska A, et al. St. Thomas modified cardioplegia effects on myoblasts' viability and morphology. Medicina (Kaunas). 2022;58(2):280.
https://doi.org/10.3390/medicina58020280
Misra S, Rao V, Choudhary A, et al. Myocardial protection in adult cardiac surgery with del Nido versus blood cardioplegia: a systematic review and meta-analysis. Heart Lung Circ. 2021;30(5):642–55.
https://doi.org/10.1016/j.hlc.2020.10.016
Ali B, Ahmed R, Khan M, et al. Comparison of routine del Nido cardioplegia vs two types of modified del Nido cardioplegias for myocardial protection among patients undergoing coronary artery bypass grafting (CABG) surgeries: a randomized double-blind clinical trial. J Extra Corpor Technol. 2024;56(3):84–93.
https://doi.org/10.1051/ject/2024011
Yang T, Liu P, Zhang D, et al. AP39 inhibits ferroptosis by inhibiting mitochondrial autophagy through the PINK1/Parkin pathway to improve myocardial fibrosis with myocardial infarction. Biomed Pharmacother. 2023;165:115195.
https://doi.org/10.1016/j.biopha.2023.115195
Le DE, Zhao Y, Kaul S. Persistent coronary vasomotor tone during myocardial ischemia occurs at the capillary level and may involve pericytes. Front Cardiovasc Med. 2022;9:930492.
https://doi.org/10.3389/fcvm.2022.930492
Zakharova VP, Sokolova NA, Ivanova AV, et al. The reaction of myocardial capillaries to crystalloid cardioplegia of different durations in patients with valvular pathology and coronary heart disease. Ukr J Cardiovasc Surg. 2022;30(4):39–46.
https://doi.org/10.30702/ujcvs/22.30(04)/ZK065-3946
Lira KB, Almeida JF, Ferreira L, et al. Myocardial protection: comparing histological effects of single-dose cardioplegic solutions—study protocol for a secondary analysis of the CARDIOPLEGIA trial. J Thorac Dis. 2024;16(2):1480–7.
https://doi.org/10.21037/jtd-23-1442
Sen O, Keles C, Ozyalcin S, et al. Custodiol versus blood cardioplegia: comparison of myocardial immunohistochemical analysis and clinical outcomes. Braz J Cardiovasc Surg. 2022;37:680–7.
https://doi.org/10.21470/1678-9741-2020-0662
Çayır MÇ, Kaya AD, Alşalaldeh M. Comparison of the cardioprotective effects of St. Thomas and del Nido cardioplegia. Cardiovasc Surg Interv. 2023;10(1):125–33.
https://doi.org/10.5606/e-cvsi.2023.1538
Osorio-Llanes E, Pérez R, Cedeño M, et al. Novel strategies to improve the cardioprotective effects of cardioplegia. Curr Cardiol Rev. 2024;20(1):39–52.
https://doi.org/10.2174/011573403X263956231129064455
Yamashita Y, et al. Effect of del Nido cardioplegia on isolated coronary artery bypass grafting: a study-level meta-analysis. J Cardiothorac Vasc Anesth. 2025;(Ahead of print).
https://doi.org/10.1053/j.jvca.2025.01.007
Aronowitz DI, Brown G, Brigham K, et al. Serum lidocaine levels in adult patients undergoing cardiac surgery with del Nido cardioplegia. Ann Thorac Surg Short Rep. 2024;2(2):302–5.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Burak Toprak, Abdulkadir Bilgiç, Murat Özeren, Ebru Ballı

This work is licensed under a Creative Commons Attribution 4.0 International License.









