Tubular functional capacity and maladaptive parathyroid hormone response in early-stage chronic kidney disease
DOI:
https://doi.org/10.17305/bb.2025.13395Keywords:
Tubular functional capacity, effective renal plasma flow, glomerular filtration rate, intact parathyroid hormone, chronic kidney failureAbstract
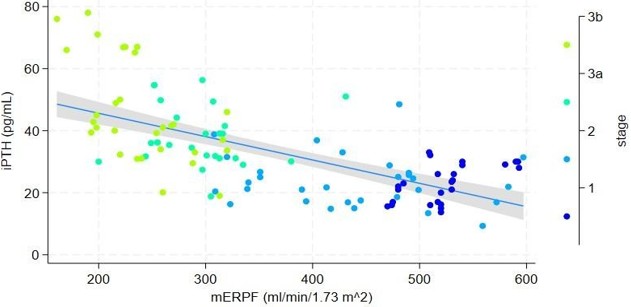
Clinical data regarding the interaction between tubular functional capacity (TFC) and maladaptive parathyroid gland response in early-stage chronic kidney disease (CKD) are limited. This study aimed to evaluate the association between parathyroid gland response, measured as intact parathyroid hormone (iPTH) serum concentration (pg/mL) using chemiluminescent microparticle immunoassay, and the dissociation between the decline in glomerular filtration rate (GFR) and TFC, assessed through radionuclide clearances. TFC was evaluated by measuring effective renal plasma flow (mERPF, ml/min/1.73m²) using (131I) Hippurate (131I-H) clearance, while GFR was measured using (99m) Tc-DTPA (mGFR, ml/min/1.73m²). Consecutive participants with preexisting CKD (N=111, female 44%, male 56%) were enrolled and stratified into four groups based on CKD stages (1, 2, 3a, and 3b). Median serum iPTH concentrations significantly differed between Stage 1 [23 (20.4-25.5) pg/mL] and Stage 2 [23.6 (20.5-26.8) pg/mL] compared to Stage 3a [38.1 (34.1-41.9) pg/mL] and Stage 3b [45.8 (39.7-51.9) pg/mL] (p=0.01). In Stage 1, there was a significant positive association between iPTH and mERPF (p=0.003). Conversely, in Stage 3b, iPTH was significantly negatively associated with both mGFR and mERPF (p<0.05 for both). Regression models that included the interaction between CKD stage and either mGFR or mERPF, alongside other predictors (age, CKD stage, body mass index, ionized calcium, and 25-hydroxyvitamin D), revealed significant associations with iPTH (p<0.05 for all variables). The assessment of TFC using 131I-H plasma clearance does not enhance the detection of maladaptive parathyroid gland responses compared to evaluating CKD stage and its relationship with declining glomerular and tubular clearances in early-stage CKD patients.
Citations
Downloads
References
Deluque AL, Dimke H, Alexander RT. Biology of calcium homeostasis regulation in intestine and kidney. Nephrol Dial Transplant. 2025;40(3):435–45. https://doi.org/10.1093/ndt/gfae204
Li X, Lu Y, Zhang L, Song A, Zhang H, Pang B, et al. Primary and secondary hyperparathyroidism present different expressions of calcium-sensing receptor. BMC Surg. 2023;23(1):31. https://doi.org/10.1186/s12893-023-01928-5
Palumbo VD, Palumbo VD, Damiano G, Messina M, Fazzotta S, Lo Monte G, et al. Tertiary hyperparathyroidism: a review. Clin Ter. 2021;172(3):241–6.
Singh P, Bhadada SK, Dahiya D, Arya AK, Saikia UN, Sachdeva N, et al. Reduced Calcium Sensing Receptor (CaSR) Expression Is Epigenetically Deregulated in Parathyroid Adenomas. J Clin Endocrinol Metab. 2020;105(9):3015–24. https://doi.org/10.1210/clinem/dgaa419
Arya AK, Kumari P, Singh P, Bhadada SK. Molecular basis of symptomatic sporadic primary hyperparathyroidism: New frontiers in pathogenesis. Best Pract Res Clin Endocrinol Metab. 2025;39(2):101985. https://doi.org/10.1016/j.beem.2025.101985
Lemoine S, Figueres L, Bacchetta J, Frey S, Dubourg L. Calcium homeostasis and hyperparathyroidism: Nephrologic and endocrinologic points of view. Ann Endocrinol (Paris). 2022;83(4):237–43. https://doi.org/10.1016/j.ando.2022.05.003
Tsai SH, Kan WC, Jhen RN, Chang YM, Kao JL, Lai HY, et al. Secondary hyperparathyroidism in chronic kidney disease: A narrative review focus on therapeutic strategy. Clin Med (Lond). 2024;24(5):100238. https://doi.org/10.1016/j.clinme.2024.100238
Kushner P, Khunti K, Cebrián A, Deed G. Early Identification and Management of Chronic Kidney Disease: A Narrative Review of the Crucial Role of Primary Care Practitioners. Adv Ther. 2024;41(10):3757–70. https://doi.org/10.1007/s12325-024-02957-z
Bozic M, Diaz-Tocados JM, Bermudez-Lopez M, Forné C, Martinez C, Fernandez E, et al. Independent effects of secondary hyperparathyroidism and hyperphosphataemia on chronic kidney disease progression and cardiovascular events: an analysis from the NEFRONA cohort. Nephrol Dial Transplant. 2022;37(4):663–72. https://doi.org/10.1093/ndt/gfab184
Khundmiri SJ, Murray RD, Lederer E. PTH and Vitamin D. Compr Physiol. 2016;6(2):561–601. https://doi.org/10.1002/j.2040-4603.2016.tb00690.x
Ulmer CZ, Kritmetapak K, Singh RJ, Vesper HW, Kumar R. High-Resolution Mass Spectrometry for the Measurement of PTH and PTH Fragments: Insights into PTH Physiology and Bioactivity. J Am Soc Nephrol. 2022;33(8):1448–58. https://doi.org/10.1681/ASN.2022010036
Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, et al. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2009;4(1):186–94. https://doi.org/10.2215/CJN.03050608
van Ballegooijen AJ, Rhee EP, Elmariah S, de Boer IH, Kestenbaum B. Renal Clearance of Mineral Metabolism Biomarkers. J Am Soc Nephrol. 2016;27(2):392–7. https://doi.org/10.1681/ASN.2014121253
Martin K, Hruska K, Greenwalt A, Klahr S, Slatopolsky E. Selective uptake of intact parathyroid hormone by the liver: differences between hepatic and renal uptake. J Clin Invest. 1976;58(4):781–8. https://doi.org/10.1172/JCI108529
Dowling TC, Frye RF, Fraley DS, Matzke GR. Characterization of tubular functional capacity in humans using para-aminohippurate and famotidine. Kidney Int. 2001;59(1):295–303. https://doi.org/10.1046/j.1523-1755.2001.00491.x
KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4 Suppl):S117–S314. https://doi.org/10.1016/j.kint.2023.10.018
Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;25(8):759–69. https://doi.org/10.1097/01.mnm.0000136715.71820.4a
Blaufox MD, Merrill JP. Simplified hippuran clearance. Measurement of renal function in man with simplified hippuran clearances. Nephron. 1966;3(5):274–81. https://doi.org/10.1159/000179542
Fine EJ, Axelrod M, Gorkin J, Saleemi K, Blaufox MD. Measurement of effective renal plasma flow: a comparison of methods. J Nucl Med. 1987;28(9):1393–400.
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–11; discussion 12–3.
Schernthaner G, Erd W, Ludwig H, Sinzinger H, Höfer R. [Study of age and sex dependance in renal clearances with radioisotopes (author's transl)]. Aktuelle Gerontol. 1976;6(3):139–45.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–30. https://doi.org/10.1210/jc.2011-0385
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883
Seegmiller JC, Wolfe BJ, Albtoush N, Melena I, Gross SP, Vinovskis C, et al. Tubular Secretion Markers, Glomerular Filtration Rate, Effective Renal Plasma Flow, and Filtration Fraction in Healthy Adolescents. Kidney Med. 2020;2(5):670–2. https://doi.org/10.1016/j.xkme.2020.05.013
Alhummiany B, Sharma K, Buckley DL, Soe KK, Sourbron SP. Physiological confounders of renal blood flow measurement. Magma. 2024;37(4):565–82. https://doi.org/10.1007/s10334-023-01126-7
Wang Y, Liu J, Fang Y, Zhou S, Liu X, Li Z. Estimating the global prevalence of secondary hyperparathyroidism in patients with chronic kidney disease. Front Endocrinol (Lausanne). 2024;15:1400891. https://doi.org/10.3389/fendo.2024.1400891
Daimon M, Fujita T, Murabayashi M, Mizushiri S, Murakami H, Nishiya Y, et al. Exacerbation of Hyperparathyroidism, Secondary to a Reduction in Kidney Function, in Individuals With Vitamin D Deficiency. Frontiers in Medicine. 2020;7:221. https://doi.org/10.3389/fmed.2020.00221
Smith HW, Goldring W, Chasis H. THE MEASUREMENT OF THE TUBULAR EXCRETORY MASS, EFFECTIVE BLOOD FLOW AND FILTRATION RATE IN THE NORMAL HUMAN KIDNEY. J Clin Invest. 1938;17(3):263–78. https://doi.org/10.1172/JCI100950
Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945;24(3):388– 404. https://doi.org/10.1172/JCI101618
Durand E, Chaumet-Riffaud P, Grenier N. Functional renal imaging: new trends in radiology and nuclear medicine. Semin Nucl Med. 2011;41(1):61–72. https://doi.org/10.1053/j.semnuclmed.2010.08.003
Kumar R, Adiga A, Novack J, Etinger A, Chinitz L, Slater J, et al. The renal transport of hippurate and protein-bound solutes. Physiol Rep. 2020;8(4):e14349. https://doi.org/10.14814/phy2.14349
Kabasakal L. Technetium-99m ethylene dicysteine: a new renal tubular function agent. European Journal of Nuclear Medicine. 2000;27(3):351–7. https://doi.org/10.1007/s002590050045
Kritmetapak K, Pongchaiyakul C. Parathyroid Hormone Measurement in Chronic Kidney Disease: From Basics to Clinical Implications. Int J Nephrol. 2019;2019:5496710. https://doi.org/10.1155/2019/5496710
Irsik DL, Bollag WB, Isales CM. Renal Contributions to Age-Related Changes in Mineral Metabolism. JBMR Plus. 2021;5(10):e10517. https://doi.org/10.1002/jbm4.10517
Towler DA. Parathyroid hormone-PTH1R signaling in cardiovascular disease and homeostasis. Trends Endocrinol Metab. 2024;35(7):648–60. https://doi.org/10.1016/j.tem.2024.02.005
Robinson-Cohen C, Lutsey PL, Kleber ME, Nielson CM, Mitchell BD, Bis JC, et al. Genetic Variants Associated with Circulating Parathyroid Hormone. J Am Soc Nephrol. 2017;28(5):1553–65. https://doi.org/10.1681/ASN.2016010069
Aloia JF, Feuerman M, Yeh JK. Reference range for serum parathyroid hormone. Endocr Pract. 2006;12(2):137–44. https://doi.org/10.4158/EP.12.2.137
Yalla N, Bobba G, Guo G, Stankiewicz A, Ostlund R. Parathyroid hormone reference ranges in healthy individuals classified by vitamin D status. J Endocrinol Invest. 2019;42(11):1353–60. https://doi.org/10.1007/s40618-019-01075-w
Smit MA, van Kinschot CMJ, van der Linden J, van Noord C, Kos S. Clinical Guidelines and PTH Measurement: Does Assay Generation Matter? Endocr Rev. 2019;40(6):1468–80. https://doi.org/10.1210/er.2018-00220
McDonnell T, Söderberg M, Taal MW, Vuilleumier N, Kalra PA, group obotN- Cas. Plasma and Urinary KIM-1 in Chronic Kidney Disease: Prognostic Value, Associations with Albuminuria, and Implications for Kidney Failure and Mortality. American Journal of Nephrology. 2025. https://doi.org/10.1159/000547867
Downloads
Published
License
Copyright (c) 2025 Branislava Ilinčić, Radmila Žeravica, Romana Mijović, Esma Isenović, Dragan Burić, Dragana Žuvić, Velibor Čabarkapa

This work is licensed under a Creative Commons Attribution 4.0 International License.









