Mechanistic insights into psoriasis and type 2 diabetes mellitus comorbidity – Implications for treatment: A review
DOI:
https://doi.org/10.17305/bb.2026.13484Keywords:
Psoriasis, type 2 diabetes mellitus, comorbidity, pathogenesis, treatmentAbstract
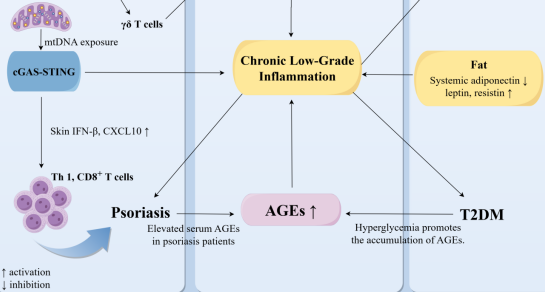
Psoriasis is a chronic systemic inflammatory disease primarily affecting the skin, yet it is increasingly recognized for its systemic implications, particularly its strong association with type 2 diabetes mellitus (T2DM). This review synthesizes recent mechanistic and clinical evidence to elucidate the shared pathways linking psoriasis and T2DM, as well as to explore therapeutic strategies for this comorbidity. We conducted a narrative review of studies published between January 2020 and October 2025, encompassing preclinical models, clinical trials, and high-quality reviews that address pathogenesis and treatment. Key findings indicate that shared genetic loci and molecular pathways, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, the IL-23/Th17 axis, and mitochondrial dysfunction associated with the activation of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, contribute to both cutaneous inflammation and systemic metabolic dysregulation. Additionally, adipokine imbalances and chronic low-grade inflammation exacerbate insulin resistance and psoriatic skin pathology. Therapeutically, IL-17/IL-23 inhibitors, metformin, glucagon-like peptide 1 (GLP-1) receptor agonists, and other immunomodulatory strategies demonstrate potential in addressing both dermatologic and metabolic features. These insights reinforce the notion of psoriasis as a systemic disorder with significant metabolic consequences, highlighting the need for integrated, multidisciplinary management. Future research should concentrate on precise gene-environment interactions, biomarker validation, and the development of treatments that simultaneously target both skin and metabolic pathology to advance precision medicine for patients with psoriasis-T2DM comorbidity.
Citations
Downloads
References
Hagenström K, Müller K, Garbe C, et al. Prevalence of psoriasis and psoriatic arthritis in Germany – analysis of claims data. J Dtsch Dermatol Ges. 2024;22(1):45–54.
https://doi.org/10.1111/ddg.15269
Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–6.
https://doi.org/10.1001/jamadermatol.2021.2007
Bellinato F, Maurelli M, Geat D, et al. Managing the patient with psoriasis and metabolic comorbidities. Am J Clin Dermatol. 2024;25(4):527–40.
https://doi.org/10.1007/s40257-024-00857-0
Yongpisarn T, Thadanipon K, Suchonwanit P, et al. Hyperglycemia is a potential prognostic factor for exacerbation in severe psoriasis with diabetes or prediabetes. Clin Cosmet Investig Dermatol. 2025;18:345–53.
https://doi.org/10.2147/CCID.S502333
Hassan FE, Aboulhoda BE, Mehesen MN, et al. Combination therapy of systemic and local metformin improves imiquimod-induced psoriasis-like lesions with type 2 diabetes: the role of AMPK/KGF/STAT3 axis. Arch Physiol Biochem. 2025;131(2):252–64.
https://doi.org/10.1080/13813455.2024.2407547
Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021.
https://doi.org/10.1530/EDM-21-0007
Yang L, Zhang L, Du Q, et al. Exploring the molecular mechanism underlying the psoriasis and T2D by using microarray data analysis. Sci Rep. 2023;13(1):19313.
https://doi.org/10.1038/s41598-023-46795-5
Pannu S, Rosmarin D. Psoriasis in patients with metabolic syndrome or type 2 diabetes mellitus: treatment challenges. Am J Clin Dermatol. 2021;22(3):293–300.
https://doi.org/10.1007/s40257-021-00590-y
Augustin J, Wolf S, Stephan B, et al. Psoriasis comorbidities in Germany: a population-based study on spatiotemporal variations. PLoS One. 2022;17(3):e0265741.
https://doi.org/10.1371/journal.pone.0265741
Lee MY, Han K, Koo HYR, et al. Psoriasis increases retinal vein occlusion risk in diabetic patients: a nationwide population-based study. Retina. 2024;44(1):151–8.
https://doi.org/10.1097/IAE.0000000000003916
Chang G, Chen B, Zhang L. Efficacy of GLP-1RA, liraglutide, in plaque psoriasis treatment with type 2 diabetes: a systematic review and meta-analysis of prospective cohort and before-after studies. J Dermatolog Treat. 2022;33(3):1299–305.
https://doi.org/10.1080/09546634.2021.1882658
Campione E, Zarabian N, Cosio T, et al. Apremilast as a potential targeted therapy for metabolic syndrome in patients with psoriasis: an observational analysis. Pharmaceuticals (Basel). 2024;17(8).
https://doi.org/10.3390/ph17080989
Alwehaidah MS, Al-Kafaji G, Bakhiet M, et al. Next-generation sequencing of the whole mitochondrial genome identifies novel and common variants in patients with psoriasis, type 2 diabetes mellitus and psoriasis with comorbid type 2 diabetes mellitus. Biomed Rep. 2021;14(5):41.
https://doi.org/10.3892/br.2021.1417
Patrick MT, Stuart PE, Zhang H, et al. Causal relationship and shared genetic loci between psoriasis and type 2 diabetes through trans-disease meta-analysis. J Invest Dermatol. 2021;141(6):1493–502.
https://doi.org/10.1016/j.jid.2020.11.025
Choudhary S, Khan NS, Verma R, et al. Exploring the molecular underpinning of psoriasis and its associated comorbidities through network approach: cross talks of genes and pathways. 3 Biotech. 2023;13(5):130.
https://doi.org/10.1007/s13205-023-03533-y
Bao L, Li J, Perez White BE, et al. Inhibition of dipeptidyl-peptidase 4 induces upregulation of the late cornified envelope cluster in keratinocytes. Arch Dermatol Res. 2022;314(9):909–15.
https://doi.org/10.1007/s00403-021-02249-4
ang W, Huang Q, Han L, et al. B7-H4 polymorphism influences the prevalence of diabetes mellitus and pro-atherogenic dyslipidemia in patients with psoriasis. J Clin Med. 2022;11(21).
https://doi.org/10.3390/jcm11216235
Watts K, Wills C, Madi A, et al. Genetic variation in ST6GAL1 is a determinant of capecitabine and oxaliplatin induced hand-foot syndrome. Int J Cancer. 2022;151(6):957–66.
https://doi.org/10.1002/ijc.34046
Alwehaidah MS, Bakhiet M, Alfadhli S. Mitochondrial haplogroup reveals the genetic basis of diabetes mellitus type 2 comorbidity in psoriasis. Med Princ Pract. 2021;30(1):62–8.
https://doi.org/10.1159/000509937
Chalitsios CV, Meena D, Manou M, et al. Multiple long-term conditions in people with psoriasis: a latent class and bidirectional Mendelian randomization analysis. Br J Dermatol. 2024;190(3):364–73.
https://doi.org/10.1093/bjd/ljad410
Li J, Li M, Kong S, et al. Bidirectional Mendelian randomization study identifies no genetic link between psoriasis and diabetes. J Diabetes Res. 2025;2025:9917071.
https://doi.org/10.1155/jdr/9917071
Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33(3):1428–34.
https://doi.org/10.1080/09546634.2020.1826392
Yao Y, Chen LQ, Lv YB, et al. Skin immune microenvironment in psoriasis: from bench to bedside. Front Immunol. 2025;16:1643418.
https://doi.org/10.3389/fimmu.2025.1643418
Lawler W, Castellanos T, Engel E, et al. Impact of obesity on the CCR6-CCL20 axis in epidermal γδ T cells and IL-17A production in murine wound healing and psoriasis. J Immunol. 2025;214(1):153–66.
https://doi.org/10.1093/jimmun/vkae011
Ma SH, Wu CY, Lyu YS, et al. Association between sodium-glucose co-transporter 2 inhibitors and risk of psoriasis in patients with diabetes mellitus: a nationwide population-based cohort study. Clin Exp Dermatol. 2022;47(12):2242–50.
https://doi.org/10.1111/ced.15385
Wen S, Liu C, Li Y, et al. Psoriasis exacerbates the state of insulin resistance in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2021;14:2389–97.
https://doi.org/10.2147/DMSO.S312420
Rajendran S, Quesada-Masachs E, Zilberman S, et al. IL-17 is expressed on beta and alpha cells of donors with type 1 and type 2 diabetes. J Autoimmun. 2021;123:102708.
https://doi.org/10.1016/j.jaut.2021.102708
Mills KH. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 2023;23(1):38–54.
https://doi.org/10.1038/s41577-022-00746-9
Sieminska I, Pieniawska M, Grzywa TM. The immunology of psoriasis – current concepts in pathogenesis. Clin Rev Allergy Immunol. 2024;66(2):164–91.
https://doi.org/10.1007/s12016-024-08991-7
Ramessur R, Corbett M, Marshall D, et al. Biomarkers of disease progression in people with psoriasis: a scoping review. Br J Dermatol. 2022;187(4):481–93.
https://doi.org/10.1111/bjd.21627
Olbrich H, Kridin K, Zirpel H, et al. GLP-1RA and reduced mortality, cardiovascular and psychiatric risks in psoriasis: a large-scale cohort study. Br J Dermatol. 2025.
https://doi.org/10.1093/bjd/ljaf346
Mustata ML, Neagoe CD, Ionescu M, et al. Clinical implications of metabolic syndrome in psoriasis management. Diagnostics (Basel). 2024;14(16).
https://doi.org/10.3390/diagnostics14161774
Xiaohong L, Zhenting Z, Yunjie Y, et al. Activation of the STING-IRF3 pathway involved in psoriasis with diabetes mellitus. J Cell Mol Med. 2022;26(8):2139–51.
https://doi.org/10.1111/jcmm.17236
Maurelli M, Gisondi P, Girolomoni G. Advanced glycation end products and psoriasis. Vaccines (Basel). 2023;11(3).
https://doi.org/10.3390/vaccines11030617
Kreouzi M, Theodorakis N, Nikolaou M, et al. Skin microbiota: mediator of interactions between metabolic disorders and cutaneous health and disease. Microorganisms. 2025;13(1).
https://doi.org/10.3390/microorganisms13010161
Kumar R, Seth S, Bhargav A, et al. Computational identification of perturbed pathways in type 2 diabetes mellitus patients reveals necroptosis and NF-κB pathways with potential for susceptibility to psoriasis. J Biosci. 2025;50.
https://doi.org/10.1007/s12038-025-00551-1
Shetty S, Wu Y, Lloyd CZ, et al. Anti-cytokine active immunotherapy based on supramolecular peptides for alleviating IL-1β-mediated inflammation. Adv Healthc Mater. 2025;14(5):e2401444.
https://doi.org/10.1002/adhm.202401444
Reddy VKK, Shiddapur G, Jagdale N, et al. Investigating interleukin-6 levels in type 2 diabetes mellitus patients with and without diabetic nephropathy. Cureus. 2024;16(8):e67014.
https://doi.org/10.7759/cureus.67014
Ren HM, Lukacher AE, Rahman ZSM, et al. New developments implicating IL-21 in autoimmune disease. J Autoimmun. 2021;122:102689.
https://doi.org/10.1016/j.jaut.2021.102689
Yang Y, Yu M, Ren L, et al. Design, synthesis and characterization of a novel multicomponent salt of bexarotene with metformin and application in ameliorating psoriasis with T2DM. Int J Pharm. 2023;646:123501.
https://doi.org/10.1016/j.ijpharm.2023.123501
Huang S, Xie K, Li X, et al. The role of the STING inflammatory pathway in hepatic damage in psoriasis with type 2 diabetes mellitus. Arch Med Sci. 2024;20(5):1426–41.
https://doi.org/10.5114/aoms/183672
Gkrinia EMM, Belančić A. The mechanisms of chronic inflammation in obesity and potential therapeutic strategies: a narrative review. Curr Issues Mol Biol. 2025;47(5).
https://doi.org/10.3390/cimb47050357
Srikanth M, Rasool M. Resistin – a plausible therapeutic target in the pathogenesis of psoriasis. Immunol Invest. 2024;53(2):115–59.
https://doi.org/10.1080/08820139.2023.2288836
Bell DS H, Jerkins T. In praise of pioglitazone: an economically efficacious therapy for type 2 diabetes and other manifestations of the metabolic syndrome. Diabetes Obes Metab. 2023;25(11):3093–102.
https://doi.org/10.1111/dom.15222
Greve AM, Wulff AB, Bojesen SE, et al. Elevated plasma triglycerides increase the risk of psoriasis: a cohort and Mendelian randomization study. Br J Dermatol. 2024;191(2):209–15.
https://doi.org/10.1093/bjd/ljae089
Hao Y, Zhou P, Zhu YJ, et al. Gut microbiota dysbiosis and altered bile acid catabolism lead to metabolic disorder in psoriasis mice. Front Microbiol. 2022;13:853566.
https://doi.org/10.3389/fmicb.2022.853566
Crane ED, Wong W, Zhang H, et al. AMPK inhibits mTOR-driven keratinocyte proliferation after skin damage and stress. J Invest Dermatol. 2021;141(9):2170–7.e3.
https://doi.org/10.1016/j.jid.2020.12.036
Huang YT, Chiu LY, Lu PH, et al. AMPK activation modulates IL-36-induced inflammatory responses by regulating IκBζ expression in the skin. Br J Pharmacol. 2024;181(15):2429–42.
https://doi.org/10.1111/bph.16354
Oh J, Han K, Doh JY, et al. High level of gamma-glutamyltransferase is a possible risk factor for psoriasis: a nationwide population-based cohort study. Indian J Dermatol Venereol Leprol. 2025;91(1):23–30.
https://doi.org/10.25259/IJDVL_42_2023
Bai C, Zhang M, Zhang Y, et al. Gamma-glutamyltransferase activity (GGT) is a long-sought biomarker of redox status in blood circulation: a retrospective clinical study of 44 types of human diseases. Oxid Med Cell Longev. 2022;2022:8494076.
https://doi.org/10.1155/2022/8494076
Baek JO, Byamba D, Wu WH, et al. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch Dermatol Res. 2012;304(9):699–706.
https://doi.org/10.1007/s00403-012-1272-y
Hsieh CY, Tseng YH, Tsai TF. Predictors for the effectiveness of 75 mg risankizumab in treating psoriasis – a real-world evidence from a 52-week retrospective study. Exp Dermatol. 2023;32(12):2138–48.
https://doi.org/10.1111/exd.14963
Zhao L, Sun L, Yang K, et al. Diabetes is associated with a poor prognosis in patients with psoriasis and coronary artery disease. BMC Endocr Disord. 2025;25(1):174.
https://doi.org/10.1186/s12902-025-01996-z
Egeberg A, Merola JF, Schäkel K, et al. Efficacy of ixekizumab in patients with moderate-to-severe plaque psoriasis and prediabetes or type 2 diabetes. Front Med (Lausanne). 2022;9:1092688.
https://doi.org/10.3389/fmed.2022.1092688
Bielach-Bazyluk A, Bossowski F, Skorupska M, et al. Psoriasis in obese adolescents with diabetes – from common molecular background to vicious circle of metabolic syndrome: case report and review of literature. Cells. 2025;14(8).
https://doi.org/10.3390/cells14080610
Thijs S, Balti E, Degraeve C, et al. Isomorphic (Koebner) phenomenon induced by insulin analogue injections in psoriasis. JCEM Case Rep. 2023;1(1):luac016.
https://doi.org/10.1210/jcemcr/luac016
Petković-Dabić J, Binić I, Carić B, et al. Effects of semaglutide treatment on psoriatic lesions in obese patients with type 2 diabetes mellitus: an open-label, randomized clinical trial. Biomolecules. 2025;15(1).
https://doi.org/10.3390/biom15010046
Huang PJ, Wei JC, Liu YT, et al. Association between α-glucosidase inhibitor use and psoriatic disease risk in patients with type 2 diabetes mellitus: a population-based cohort study. Int J Clin Pract. 2021;75(11):e14819.
https://doi.org/10.1111/ijcp.14819
Al Bahadly WKY, Bdioui A, Al-Gazally M, et al. The effect of dapagliflozin ointment on induced psoriasis in an experimental model. J Med Life. 2024;17(3):281–5.
https://doi.org/10.25122/jml-2023-0084
Ma KS, Tsai SY, Holt A, et al. Effects of sodium-glucose cotransporter-2 inhibitors on inflammatory skin diseases in patients with type 2 diabetes. J Am Acad Dermatol. 2024;91(5):934–6.
https://doi.org/10.1016/j.jaad.2024.04.079
Lynch M, Malara A, Timoney I, et al. Sitagliptin and narrow-band ultraviolet-B for moderate psoriasis (DINUP): a randomised controlled clinical trial. Dermatology. 2022;238(1):140–7.
https://doi.org/10.1159/000514494
Chen P, Chen X, Lei L, et al. The efficacy and safety of pioglitazone in psoriasis vulgaris: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(32):e21549.
https://doi.org/10.1097/MD.0000000000021549
Zhao M, Huang F, Tang L, et al. Case report: successful treatment of acute generalized pustular psoriasis with multiple comorbidities with oral tacrolimus. Front Immunol. 2024;15:1354578.
https://doi.org/10.3389/fimmu.2024.1354578
Pelosi E. Effect of oral enzyme combination, diet and exercise on chronic low-grade inflammatory conditions – a report of three cases. AME Case Rep. 2023;7:7.
https://doi.org/10.21037/acr-22-45
Tsiogka A, Gregoriou S, Stratigos A, et al. The impact of treatment with IL-17/IL-23 inhibitors on subclinical atherosclerosis in patients with plaque psoriasis and/or psoriatic arthritis: a systematic review. Biomedicines. 2023;11(2).
https://doi.org/10.3390/biomedicines11020318
Chi CC, Lee CY, Liu CY, et al. Effects of antidiabetic drugs on psoriasis: a meta-analysis. Eur J Clin Invest. 2021;51(2):e13377.
https://doi.org/10.1111/eci.13377
Paschou IA, Sali E, Paschou SA, et al. GLP-1RAs in patients with psoriasis. Hormones (Athens). 2025;24(2):291–3.
https://doi.org/10.1007/s42000-025-00635-5
Nicolau J, Nadal A, Sanchís P, et al. Effects of liraglutide among patients living with psoriasis and obesity. Med Clin (Barc). 2023;161(7):293–6.
https://doi.org/10.1016/j.medcli.2023.05.021
Tassavor B, Al Salem S. Use of glucagon-like peptide-1 (GLP-1) agonists in modulating preexisting dermatologic disease: a systematic review. Cureus. 2025;17(9):e93282.
https://doi.org/10.7759/cureus.93282
Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107:124–8.
https://doi.org/10.1016/j.yexmp.2019.01.014
Lai Y, Jiang Z, Qiu X, et al. Metformin treatment protects mice from glucose-promoted psoriasiform dermatitis through enrichment of Akkermansia muciniphila with modulation of bacterial tryptophan metabolites. J Invest Dermatol. 2025.
https://doi.org/10.1016/j.jid.2025.08.017
Zhang MX, Zheng BY, Chen HX, et al. Clinical effects of antidiabetic drugs on psoriasis: the perspective of evidence-based medicine. World J Diabetes. 2021;12(8):1141–5.
https://doi.org/10.4239/wjd.v12.i8.1141
Guo Q, Jin Y, Chen X, et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9(1):53.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2026 Ling Ouyang, Zhanzong Li, Yunhong Zeng

This work is licensed under a Creative Commons Attribution 4.0 International License.









