Oral Acute Toxicity of Polyenylphosphatidylcholine (PPC) in Rats
DOI:
https://doi.org/10.17305/bjbms.2005.3273Keywords:
polyenylphosphatidylcholine, acute oral toxicityAbstract
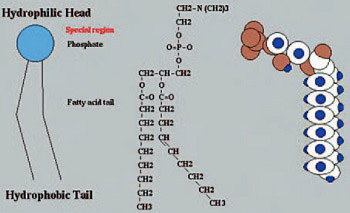
Endogen phospholipids play a major role in determining the structure and nature of cell membranes. A deficiency of phospholipids in cellular membranes makes it almost impossible for the cell membrane to perform its function as a selective barrier between what passes in and out of the cell. Polyenylphosphatidylcholine chemical structure corresponds to that of endogen phospholipids, but it possesses functional superiority because of its content of unsaturated fatty acids. Polyenylphosphatidylcholine integrates in the cell membrane and organelle systems while becoming their constitutive elements. A healthy cell membrane leads to healthy cells and then healthy tissue and then to healthy organs or body systems and finally, healthy bodies and minds. For a long time, polyenylphosphatidylcholine in combination with vitamins has been used in the treatment of numerous health problems such as liver diseases, dyslipoproteinaemias and different intoxications with consequent liver failure. The main aim of toxicology studies is evaluation of the toxic potential and risks of human exposition to the substance. According to the Organization for Economic Cooperation and Development (OECD) acute oral toxicity refers to those adverse effects occurring following oraladministration of a single dose of a substance or multiple doses given within 24 hours. LD50 (median lethal dose), oral, is a statistically derived single dose of a substance that can be expected to cause death in 50 per cent of animals when administered by the oral route. Our acute toxicity study was performed on albino Wistar rats. Animals were randomised in three experimental and one control group, each of 5 males and 5 females. Study was based on the administration of a single oral dose of the test substance (polyenylphosphatidylcholine) to each experimental animal. There were three dose-levels of the test substance: 300, 500 and 1000 mg/kg. Test substance administration day was the first day of the observation period that lasted 14 days. Control animals were given milk vehicle. At the end of the study, no statistically significant differences between experimental and control animals were observed concerning the recorded parameters: body weight, respiratory rate, tremor, faeces and phonation quality, indicating the absence of the test substance acute toxicity.
Citations
Downloads
Downloads
Published
How to Cite
Accepted 2018-03-01
Published 2005-08-20









