CAR-macrophage versus CAR-T for solid tumors: The race between a rising star and a superstar
DOI:
https://doi.org/10.17305/bb.2023.9675Keywords:
CAR-macrophage, chimeric antigen receptor T cell (CAR-T), chimeric antigen receptor (CAR), adoptive cell therapy (ACT), tumor immunotherapy, solid tumor, tumor microenvironment (TME)Abstract
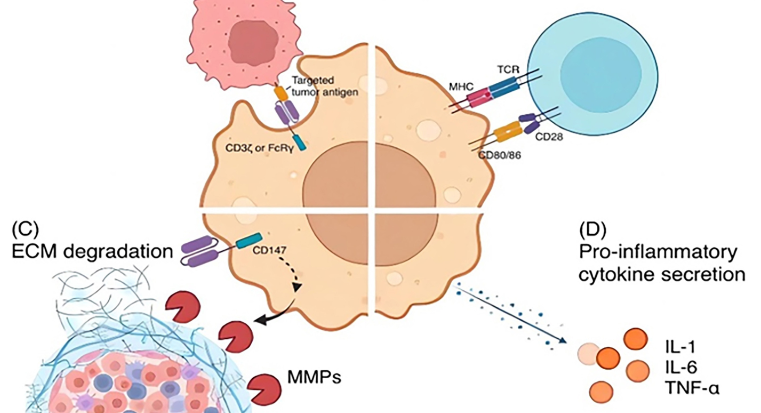
Adoptive cell therapy (ACT) has been demonstrated to be one of the most promising cancer immunotherapy strategies due to its active antitumor capabilities in vivo. Engineering T cells to overexpress chimeric antigen receptors (CARs), for example, has shown potent efficacy in the therapy of some hematologic malignancies. However, the efficacy of chimeric antigen receptor T cell (CAR-T) therapy against solid tumors is still limited due to the immunosuppressive tumor microenvironment (TME) of solid tumors, difficulty in infiltrating tumor sites, lack of tumor-specific antigens, antigen escape, and severe side effects. In contrast, macrophages expressing CARs (CAR-macrophages) have emerged as another promising candidate in immunotherapy, particularly for solid tumors. Now at its nascent stage (with only one clinical trial progressing), CAR-macrophage still shows inspiring potential advantages over CAR-T in treating solid tumors, including more abundant antitumor mechanisms and better infiltration into tumors. In this review, we discuss the relationships and differences between CAR-T and CAR-macrophage therapies in terms of their CAR structures, antitumor mechanisms, challenges faced in treating solid tumors, and insights gleaned from clinical trials and practice for solid tumors. We especially highlight the potential advantages of CAR-macrophage therapy over CAR-T for solid tumors. Understanding these relationships and differences provides new insight into possible optimization strategies of both these two therapies in solid tumor treatment.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2023 Kun Chen, Min-ling Liu, Jian-cheng Wang, Shuo Fang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-10-12
Published 2024-05-02









