Neuroprotective effects of sildenafil in experimental spinal cord injury in rabbits
DOI:
https://doi.org/10.17305/bjbms.2015.1.119Keywords:
caspase-3, gelsolin, methylprednisolone, phosphodiesterase type 5, traumaAbstract
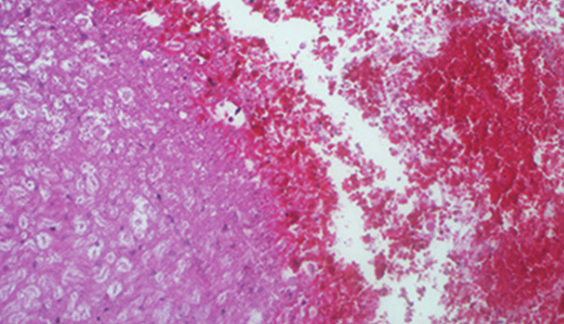
Neuroprotective agents such as methylprednisolone and sildenafil may limit damage after spinal cord injury. We evaluated the effects of methylprednisolone and sildenafil on biochemical and histologic changes after spinal cord injury in a rabbit model. Female New Zealand rabbits (32 rabbits) were allocated to 4 equal groups: laminectomy only (sham control) or laminectomy and spinal trauma with no other treatment (trauma control) or treatment with either methylprednisolone or sildenafil. Gelsolin and caspase-3 levels in cerebrospinal fluid and plasma were determined, and spinal cord histology was evaluated at 24 hours after trauma. There were no differences in mean cerebrospinal fluid or plasma levels of caspase-3 between the groups or within the groups from 0 to 24 hours after injury. From 0 to 24 hours after trauma, mean cerebrospinal fluid gelsolin levels significantly increased in the sildenafil group and decreased in the sham control and the trauma control groups. Mean plasma gelsolin level was significantly higher at 8 and 24 hours after trauma in the sildenafil than other groups. Histologic examination indicated that general structural integrity was better in the methylprednisolone in comparison with the trauma control group. General structural integrity, leptomeninges, white and grey matter hematomas, and necrosis were significantly improved in the sildenafil compared with the trauma control group. Caspase-3 levels in the cerebrospinal fluid and blood were not increased but gelsolin levels were decreased after spinal cord injury in trauma control rabbits. Sildenafil caused an increase in gelsolin levels and may be more effective than methylprednisolone at decreasing secondary damage to the spinal cord.
Citations
Downloads
References
Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 2008; 25:E2.
Gál P, Kravcuková P, Mokrý M, Kluchová D. Chemokines as possible targets in modulation of the secondary damage after acute spinal cord injury: a review. Cell Mol Neurobiol 2009;29:1025-1035.
http://dx.doi.org/10.1007/s10571-009-9392-4
Toklu HZ, Hakan T, Celik H, Biber N, Erzik C, Ogunc AV, et al. Neuroprotective effects of alpha-lipoic acid in experimental spinal cord injury in rats. J Spinal Cord Med 2010; 33:401-409.
Erol FS, Kaplan M, Tiftikci M, Yakar H, Ozercan I, Ilhan N, et al. Comparison of the effects of octreotide and melatonin in preventing nerve injury in rats with experimental spinal cord injury. J Clin Neurosci 2008; 15:784-790.
http://dx.doi.org/10.1016/j.jocn.2007.06.009
Barut S, Unlu YA, Karaoglan A, Tuncdemir M, Dagistanli FK, Ozturk M, et al. The neuroprotective effects of z-DEVD.fmk, a caspase-3 inhibitor, on traumatic spinal cord injury in rats. Surg Neurol 2005; 64:213-220.
http://dx.doi.org/10.1016/j.surneu.2005.03.042
Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, et al. Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 2000; 166:213-226.
http://dx.doi.org/10.1006/exnr.2000.7523
Solaroglu I, Kaptanoglu E, Okutan O, Beskonakli E, Attar A, Kilinc K. Magnesium sulfate treatment decreases caspase-3 activity after experimental spinal cord injury in rats. Surg Neurol 2005; 64(suppl 2):S17-S21.
http://dx.doi.org/10.1016/j.surneu.2005.07.058
Li GH, Arora PD, Chen Y, McCulloch CA, Liu P. Multifunctional roles of gelsolin in health and diseases. Med Res Rev 2012; 32:999-1025.
http://dx.doi.org/10.1002/med.20231
Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci 2004; 61:2614-2623.
http://dx.doi.org/10.1007/s00018-004-4225-6
Bucki R, Levental I, Kulakowska A, Janmey PA. Plasma gelsolin: function, prognostic value, and potential therapeutic use. Curr Protein Pept Sci 2008; 9:541-551.
http://dx.doi.org/10.2174/138920308786733912
Le HT, Hirko AC, Thinschmidt JS, Grant M, Li Z, Peris J, et al. The protective effects of plasma gelsolin on stroke outcome in rats. Exp Transl Stroke Med 2011; 3:13.
http://dx.doi.org/10.1186/2040-7378-3-13
Peddada N, Sagar A, Ashish, Garg R. Plasma gelsolin: a general prognostic marker of health. Med Hypotheses 2012; 78:203-210.
http://dx.doi.org/10.1016/j.mehy.2011.10.024
Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx 2004; 1:80-100.
http://dx.doi.org/10.1602/neurorx.1.1.80
Kerimoglu A, Pasaoglu O, Kanbak G, Hanci V, Ozdemir F, Atasoy MA. Efficiency of coenzyme Q(10) at experimental spinal cord injury [in Turkish]. Ulus Travma Acil Cerrahi Derg 2007; 13:85-93.
Farooq MU, Naravetla B, Moore PW, Majid A, Gupta R, Kassab MY. Role of sildenafil in neurological disorders. Clin Neuropharmacol 2008; 31:353-362.
http://dx.doi.org/10.1097/WNF.0b013e31815cd94c
Atalay B, Caner H, Cekinmez M, Ozen O, Celasun B, Altinors N. Systemic administration of phosphodiesterase V inhibitor, sildenafil citrate, for attenuation of cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery 2006; 59:1102-1107.
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 15 December 2013.
Minitab, Inc. (2003) Minitab 14 (statistical software). Minitab, Inc., State College, PA
Kalayci M, Coskun O, Cagavi F, Kanter M, Armutcu F, Gul S, et al. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem Res 2005; 30:403-410.
http://dx.doi.org/10.1007/s11064-005-2615-2
Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res 2011; 1374:100-109.
http://dx.doi.org/10.1016/j.brainres.2010.11.061
Kurt G, Ergun E, Cemil B, Borcek AO, Borcek P, Gulbahar O, et al. Neuroprotective effects of infliximab in experimental spinal cord injury. Surg Neurol 2009; 71:332-336.
http://dx.doi.org/10.1016/j.surneu.2008.01.038
Kahraman S, Duz B, Kayali H, Korkmaz A, Oter S, Aydin A, et al. Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: a comparative study in rats. Neurochem Res 2007; 32:1547-1551.
http://dx.doi.org/10.1007/s11064-007-9354-5
Hall ED. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics 2011; 8:152-167.
http://dx.doi.org/10.1007/s13311-011-0026-4
Lin HS, Ji ZS, Zheng LH, Guo GQ, Chen B, Wu H, et al. Effect of methylprednisolone on the activities of caspase-3, -6, -8 and -9 in rabbits with acute spinal cord injury. Exp Ther Med 2012; 4:49-54.
Merola A, O'Brien MF, Castro BA, Smith DA, Eule JM, Lowe TG, et al. Histologic characterization of acute spinal cord injury treated with intravenous methylprednisolone. J Orthop Trauma 2002; 16:155-161.
http://dx.doi.org/10.1097/00005131-200203000-00003
Baptiste DC, Fehlings MG. Emerging drugs for spinal cord injury. Expert Opin Emerg Drugs 2008; 13:63-80.
http://dx.doi.org/10.1517/14728214.13.1.63
Nakamizo T, Kawamata J, Yoshida K, Kawai Y, Kanki R, Sawada H, et al. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res 2003; 71:485-495.
http://dx.doi.org/10.1002/jnr.10483
Raposo C, Nunes AK, Luna RL, Araújo SM, da Cruz-Höfling MA, Peixoto CA. Sildenafil (Viagra) protective effects on neuroinflammation: the role of iNOS/NO system in an inflammatory demyelination model. Mediators Inflamm 2013; 2013:321460.
http://dx.doi.org/10.1155/2013/321460
Kiymaz N, Yilmaz N, Mumcu C, Anlar O, Ozen S, Kayaoglu CR. Protective effect of sildenafil (Viagra) in transient spinal cord ischemia. Pediatr Neurosurg 2008; 44:22-28.
http://dx.doi.org/10.1159/000110658
Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH. Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res 1998; 10:69-73.
http://dx.doi.org/10.1038/sj.ijir.3900354
Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract 2006; 60:967-975.
http://dx.doi.org/10.1111/j.1742-1241.2006.01049.x
D'Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci 2012; 35:700-709.
http://dx.doi.org/10.1016/j.tins.2012.06.004
Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, et al. Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience 2000; 99:333-342.
http://dx.doi.org/10.1016/S0306-4522(00)00173-1
DiNubile MJ. Plasma gelsolin as a biomarker of inflammation. Arthritis Res Ther 2008; 10:124.
http://dx.doi.org/10.1186/ar2547
Lee PS, Drager LR, Stossel TP, Moore FD, Rogers SO. Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann Surg 2006; 243:399-403.
http://dx.doi.org/10.1097/01.sla.0000201798.77133.55
Lee PS, Waxman AB, Cotich KL, Chung SW, Perrella MA, Stossel TP. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit Care Med 2007; 35:849-855.
http://dx.doi.org/10.1097/01.CCM.0000253815.26311.24
Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 1997; 17:5395-5406.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2015 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2014-10-08
Published 2015-01-08









