Neuropeptide S pathway in PTSD and neuropsychiatric disorders: A review
DOI:
https://doi.org/10.17305/bb.2025.12861Keywords:
Post-traumatic stress disorder, related neuropsychiatric disorders, neuropeptide S, neuropeptide S receptor, single nucleotide polymorphismAbstract
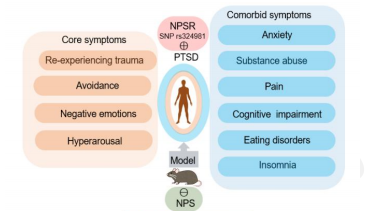
Post-traumatic stress disorder (PTSD) is a multidimensional illness that seldom occurs alone: roughly 80 % of patients also meet criteria for anxiety, depression, chronic pain, substance-use, eating or cognitive disorders. Converging genetic, neurochemical and behavioural findings implicate the neuropeptide S (NPS) system—acting through its G-protein-coupled NPS receptor (NPSR)—as a common regulator of these diverse phenotypes. This narrative review surveys studies published 2000–2024 in PubMed, Embase and Web of Science that examine NPS/NPSR involvement in core PTSD features and typical comorbidities. The functional rs324981 A/T polymorphism, which boosts NPSR surface expression and signalling, consistently associates with greater PTSD risk and symptom severity. In rodent models, exogenous NPS reduces anxiety- and fear-like behaviours, speeds fear-memory extinction, stabilises the hypothalamic-pituitary-adrenal axis, enhances dopaminergic tone and elevates hippocampal brain-derived neurotrophic factor (BDNF)—changes concordant with symptom relief. Additional work shows that NPS lessens pain affect, dampens alcohol and opioid intake, eases withdrawal-induced anxiety and lowers food consumption, hinting at a multimodal therapeutic profile. These effects converge on limbic and mid-brain circuits (amygdala, ventral tegmental area, locus coeruleus, paraventricular nucleus) and engage oxytocinergic, adenosinergic and endocannabinoid pathways. Translation remains limited by NPS’s rapid degradation, poor blood–brain-barrier penetration and scarcity of brain-penetrant NPSR ligands, but advances in intranasal delivery, lipid-acylated analogues, biased NPSR agonists and “humanised” NPSR-variant models offer promising solutions. Collectively, current pre-clinical and genetic evidence positions the NPS–NPSR axis as a versatile therapeutic target for both core PTSD symptoms and their disabling comorbidities, warranting rigorous translational studies to refine mechanism, optimise drug-like properties and test clinical efficacy.
Citations
Downloads
Downloads
Published
License
Copyright (c) 2025 Zhi-cheng Zhu, Xue-jing Han, Zhen He, Meng-yang Liu, Ning Wu, Xiang-min Tong, Fei Li

This work is licensed under a Creative Commons Attribution 4.0 International License.









