Gut microbial metabolites and the brain–gut axis in Alzheimer’s disease: A review
DOI:
https://doi.org/10.17305/bb.2025.12921Keywords:
Alzheimer's disease, brain–gut axis, gut microbial metabolites, mechanismAbstract
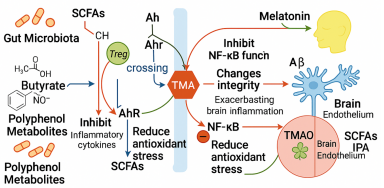
Alzheimer’s disease (AD) is increasingly recognised as a disorder that extends beyond the brain, with accumulating evidence implicating gut microbiota–derived metabolites in its onset and progression. This narrative review synthesises 92 peer-reviewed animal, human and meta-analytic studies published between 2010 and 2025 that investigated short-chain fatty acids (SCFAs), tryptophan-derived indoles and kynurenines, trimethylamine N-oxide (TMAO) and secondary bile acids in the context of AD. Collectively, the literature shows that SCFAs support blood–brain-barrier integrity, dampen microglial reactivity and enhance synaptic plasticity, yet can paradoxically amplify β-amyloid (Aβ) deposition under germ-free or supraphysiological conditions, highlighting the importance of host status and dosing. Beneficial indole metabolites such as indole-3-propionic acid counter oxidative stress, strengthen intestinal and cerebral barriers and suppress pro-inflammatory cascades, whereas a shift toward neurotoxic kynurenines correlates with cognitive decline. TMAO emerges as a consistently deleterious metabolite that aggravates endothelial dysfunction, neuroinflammation and Aβ aggregation; dietary precursor restriction and microbial enzyme inhibitors are therefore being explored as mitigation strategies. Secondary bile acids and polyphenol derivatives further modulate mitochondrial bioenergetics and NF-κB signalling, broadening the therapeutic landscape. Multi-omics profiling reveals that AD patients typically exhibit reduced SCFAs and indoles but elevated TMAO, changes that scale with Mini-Mental State Examination scores, brain atrophy and cerebrospinal Aβ₄₂ levels. Early probiotic and faecal-microbiota-transplant trials have begun to normalise these metabolite profiles and yield modest cognitive benefits, underscoring translational potential. Altogether, gut-derived metabolites are not passive by-products but active modulators of neural, immune and metabolic circuits along the microbiota–gut–brain axis; their targeted manipulation and standardised metabolomic assessment could enable earlier diagnosis and precision microbiome-based interventions for AD, a promise that now warrants validation in large, longitudinal and mechanistically informed clinical studies.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Xinchen Ji, Jian Wang, Tianye Lan, Dexi Zhao, Peng Xu

This work is licensed under a Creative Commons Attribution 4.0 International License.









