Sclerostin antibody promotes alveolar bone regeneration after tooth extraction
DOI:
https://doi.org/10.17305/bb.2025.12999Keywords:
Sclerostin-ab, bone regeneration, graft materialAbstract
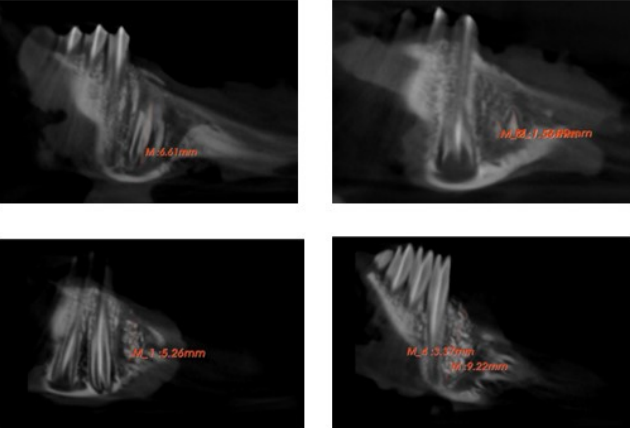
Sclerostin is a key inhibitor of the Wnt signaling pathway, functioning by binding to the LRP5/6 receptor. This interaction inhibits beta-catenin expression, resulting in the downregulation of osteogenic markers, which contributes to the promotion of osteoporosis and an increase in osteoclast numbers. The primary objective of this research was to investigate the effects of sclerostin antibody (Scl-ab) on bone formation utilizing graft materials in tooth sockets, and to analyze the regulatory interaction between sclerostin and bone tissue through targeted sclerostin inhibition and stimulation of bone formation in tooth extraction sockets following local, single-dose administration. In this study, New Zealand male rabbits (3 months old, weighing 2.5-3 kg) were fully randomized to minimize bias. The experiments were conducted across five groups: a control group, a graft group, and three experimental groups receiving 100%, 75%, and 50% doses of Scl-ab. Calculated doses of Scl-ab were administered alongside the graft material in the extraction sockets, with results assessed at 2 and 4-week intervals. Cone-beam computed tomography indicated that the tooth extraction sockets treated with varying ratios of Scl-ab with graft material exhibited a statistically significant increase in the mean mandibular BV/TV ratio compared to the control and graft groups, with variations based on time and dosage. While bone volume improved over time, the most significant enhancement was observed in the 100% Scl-ab group. Additionally, the administration of different doses of Scl-ab significantly increased trabecular thickness of the alveolar bone compared to both the control (p < 0.001) and graft (p < 0.001) groups, with histological analysis corroborating these findings. The therapeutic application of Scl-ab facilitates early bone formation, and the localized inhibition of sclerostin secreted within the bone microenvironment targets potential bone regeneration.
Citations
Downloads
References
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 2003;22:6267–6276. doi.org/10.1093/emboj/cdg599.
Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis 2014;6(2):48–57. 10.1177/1759720x13510479
Gao Y, Chen N, Fu Z, Zhang Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023;13:483. doi.org/10.3390/biom13030483.
Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 2005;280(29):26770–5. doi.org/10.1074/jbc.M504308200.
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet 2011;10:537–544. 10.1093/hmg/10.5.537.
Van Hul W, Balemans W, Van Hul E, Dikkers FG, Obee H, Stokroos RJ, et al. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21. Am J Hum Genet 1998;62:391–399.
Winkler DG, Yu C, Geoghegan JC, Ojala EW, Skonier JE, Shpektor D, et al. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem 2004; 279:36293–8. 10.1074/jbc.M400521200.
Veverka V. Henry AJ, Slocombe PM, Ventom A, Mulloy B, Muskett FW, et al. Characterization of the structural features and interactions of sclerostin. J Biol Chem 2009;284:10890–10900. 10.1074/jbc.M807994200.
Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. J Bone 2017;96:29–37. 10.1016/j.bone.2016.10.007.
Weivoda MM, Youssef SJ, Jo Oursler M. Sclerostin expression and functions beyond the osteocyte. J Bone 2017;96:45–50. 10.1016/j.bone.2016.11.024 30.
Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. Journal of Musculoskeletal & Neuronal Interactions 2006;6(4):35.
Ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signalling. J Bone Joint Surg Am 2008;90(1): 31.
Dincel AS, Jørgensen NR; IOF-IFCC Joint Committee on Bone Metabolism (C-BM). New emerging biomarkers for bone disease: Sclerostin and Dickkopf-1 (DKK1). Calcif Tissue Int 2023;112(2):243-257. doi.org/10.1007/s00223-022-01020-9.
Şimşek Y, Baran SS, Ergünol E, Uludamar A, Dinçel AS, Erkoç Ş. In silico identification of sclerostin inhibitors. Mol Divers 2025; Jul 29.doi:10.1007/s11030-025-11298-0. Epub ahead of print. PMID: 40728828.
do Amaral Couto BA, Fernandes JCH, Saavedra-Silva M, Hernan Roca H, Rogério Moraes Castilho MR, Fernandes GVO. Antisclerostin Effect on Osseointegration and Bone Remodeling J. Clin. Med. 2023, 12, 1294. doi.org/10.3390/jcm12041294
Nakashima F, Matsuda S, Ninomiya Y, Ueda T, Yasuda K, Hatano S, et al. Role of sclerostin deletion in bisphosphonate-induced osteonecrosis of the jaw. Bone 2024;187:117200.
Nascimento GG, Alves-Costa S, Romandini M. Burden of severe periodontitis and edentulism in 2021, with projections up to 2050: The Global Burden of Disease 2021 study. J Periodont Res. 2024; 59: 823-867. doi.org10.1111/jre.13337
Ergünol, E., Akgün, R.O., Şemsi, R., Ekim, O., Uludamar, A., and Dinçel, A.S. Novel Method for Tooth Extraction in Rabbits: Advantages and Considerations. Indian Journal of Animal Research 2025;1-6. doi:10.18805/IJAR.BF-1901 Epub ahead of print.
Dougherty R, Kunzelmann K. Computing local thickness of 3D structures with ImageJ. Microsc. Microanal. 2007;13:1678–1679. doi.org/10.1017/S1431927607074430
Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone. 2010 Dec;47(6):1076-9. doi.org/10.1016/j.bone.2010.08.023.
Recommended Citation: U.S. Food and Drug Administration. (2024). BoneJ Headless: An Automated Python Tool for Bone Microstructure Analysis (RST24AI03.01). https://cdrh-rst.fda.gov/bonej-headless-automated-python-tool-bone-microstructure-analysis
Ebrakhim, M.; Moiseev, D.; Strelnikov, V.; Salloum, A.; Faustova, E.; Ermolaev, A.; Enina, Y.; Velichko, E.; Vasil’ev, Y. A New Method for the Digital Assessment of the Relative Density of Bone Tissue in Dentistry Using the ImageJ Software Package. Dent. J. 2025, 13, 375. doi.org/10.3390/dj13080375
Sangeetha R, Uma K, Chandavarkar V. Comparison of routine decalcification methods with microwave decalcification of bone and teeth. J Oral Maxillofac Pathol 2013;17(3):386-91.
Sung HH, Kwon HH, Stephan C, Reynolds SM, Dai Z, Van der Kraan PM. Sclerostin antibody enhances implant osseointegration in bone with Col1a1 mutation. Bone 2024;186:117167.
Mavropoulos A, Rizzoli R, Ammann P. Different responsiveness of alveolar and tibial bone to bone loss stimuli, J. Bone Miner. Res. 2007;22: 403–410. [PubMed: 17181394]
Feng Zhu, Yong Qiu, Hiu Yan Yeung, Kwong Man Lee, Chun-yiu Jack Cheng. Trabecular bone micro-architecture and bone mineral density in adolescent idiopathic and congenital scoliosis. Orthopaedic Surgery 2009;1(1):78–83. doi.org/10.1111/j.1757-7861.2008.00014.x.
Marino S, et al. Reversal of the diabetic bone signature with anabolic therapies in mice. Bone Res. 2023;11(1):19.
Disha-Ibrahimi S, Furlani B, Drevenšek G, Hudoklin S, Marc J, Prodan Žitnik I, Sajovic J, Drevenšek M. Olanzapine decreased osteocyte maturation and Wnt/β-catenin signaling during loading of the alveolar bone in rats. Biomol Biomed 2023;1;23(1):114-125. doi.org.(10.17305/bjbms.2022.7523. PMID: 35880348; PMCID: PMC9901902.
Turkkahraman H, Flanagan S, Zhu T, Akel N, Marino S, Ortega-Gonzalez D, et al. Sclerostin antibody corrects periodontal disease in type 2 diabetic mice. JCI Insight 2024;9(16):e181940 doi.org/10.1172/jci.insight.181940 .
Taut AD, Jin Q, Chung JH, Galindo-Moreno P, Yi ES, Sugai JV, Ke HZ, Liu M, Giannobile WV. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res 2013;28(11):2347-56. doi: 10.1002/jbmr.1984. PMID: 23712325
Yao Y, Kauffmann F, Maekawa S, Sarment LV, Sugai JV, Schmiedeler CA, Doherty EJ, Holdsworth G, Kostenuik PJ, Giannobile WV. Sclerostin antibody stimulates periodontal regeneration in large alveolar bone defects. Sci Rep 2020;1,10(1):16217. 10.1038/s41598-020-73026-y.
Zhao R, Yang R, Cooper PR, Khurshid Z, Shavandi A, Ratnayake J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021;18, 26(10):3007. doi: 10.3390/molecules26103007.
Kumala ELC, Fauzia M, Junivianti HS. The effect of nanoparticle tooth grafts on osteoblast stimulation in the first stages of the bone healing process in Wistar rats compared to the micro-tooth graft technique. Dental Journal 2023;56(3):184–188. doi.org/10.20473/j.djmkg.v56.i3.p184-188
Brannigan K, Griffin M. An update into the application of nanotechnology in bone healing. Open Orthop J 2016; 10(1): 808–23.
Choi RB, Bullock WA, Hoggatt AM, Loots GG, Genetos DC, Robling AG. Improving Bone Health by Optimizing the Anabolic Action of Wnt Inhibitor Multitargeting. JBMR Plus 2021;5(5):e10462. doi.org/: 10.1002/jbm4.10462.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Erdal Ergünol, Rabia Şemsi, Duygu Dayanır, Remzi Orkun Akgün, Okan Ekim, Altay Uludamar, Ayhan Özkul, Aylin Sepici Dinçel

This work is licensed under a Creative Commons Attribution 4.0 International License.









