Acid ceramidase expression and biomarker potential in patients with locally advanced rectal cancer
DOI:
https://doi.org/10.17305/bb.2025.13275Keywords:
Acid ceramidase, ASAH1 gene, CALLY index, neoadjuvant chemoradiotherapy, rectal cancerAbstract
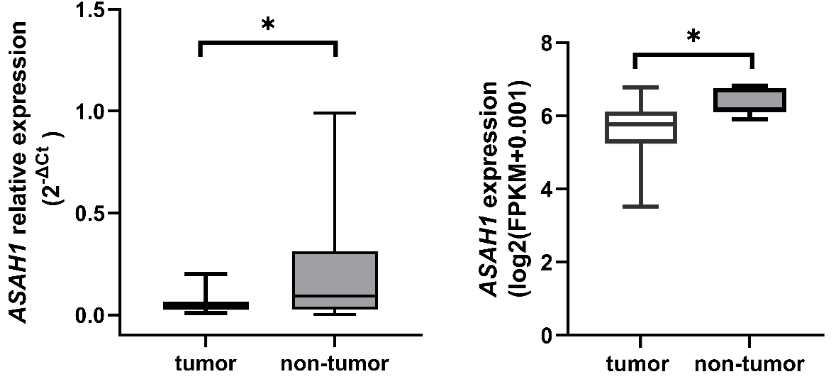
Acid ceramidase (AC), a pivotal enzyme in sphingolipid metabolism, has been associated with various cancers; however, its specific role in rectal cancer remains poorly understood. This study aimed to explore the clinical significance of AC gene and protein expression in rectal cancer. We analyzed the expression of ASAH1, BAX, and BCL2 through quantitative Real-Time PCR in paired tumor and non-tumor tissue samples obtained from patients with locally advanced rectal cancer (LARC) prior to neoadjuvant chemoradiotherapy. Additionally, serum AC levels and standard biochemical parameters were assessed. We further evaluated ASAH1 expression using RNA-seq data from publicly available TCGA-READ datasets accessed via the UCSC Xena Browser. Two approaches indicated a significant reduction in ASAH1 expression in tumor tissue (p=0.004 and p<0.001, respectively). Receiver operating characteristic curve analysis revealed a modest capacity for ASAH1 expression to differentiate between tumor and non-tumor tissue in LARC patients (AUC=0.652, p=0.042). No correlation was observed between ASAH1 expression and the BAX/BCL2 ratio in tumor tissue, nor with serum AC levels or the CRP-albumin-lymphocyte (CALLY) index. Conversely, serum AC levels exhibited a negative correlation with the BAX/BCL2 ratio (rs=−0.536, p=0.002, FDR-adjusted q=0.021). Furthermore, ASAH1 expression, AC levels, and the CALLY index were not linked to overall survival or treatment response. A key finding of this study is the inverse relationship between serum AC levels and the pro-apoptotic status of tumor tissue, suggesting that circulating AC may provide valuable insights into tumor apoptotic activity. Further large-scale studies are necessary to validate these preliminary findings and elucidate the biomarker potential of AC in rectal cancer.
Citations
Downloads
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74(3):229-63.
https://doi.org/10.3322/caac.21834
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73(3):233-54.
https://doi.org/10.3322/caac.21772
Mendis S, To YH, Tie J. Biomarkers in Locally Advanced Rectal Cancer: A Review. Clin Colorectal Cancer 2022;21(1):36-44.
https://doi.org/10.1016/j.clcc.2021.11.002
Van Cutsem E, Borràs JM, Castells A, Ciardiello F, Ducreux M, Haq A, et al. Improving outcomes in colorectal cancer: where do we go from here? Eur J Cancer 2013;49(11):2476-85.
https://doi.org/10.1016/j.ejca.2013.03.026
Camp ER, Patterson LD, Kester M, Voelkel-Johnson C. Therapeutic implications of bioactive sphingolipids: A focus on colorectal cancer. Cancer Biol Ther 2017;18(9):640-50.
https://doi.org/10.1080/15384047.2017.1345396
Gani C, Kirschniak A, Zips D. Watchful Waiting after Radiochemotherapy in Rectal Cancer: When Is It Feasible? Visc Med 2019;35(2):119-23.
https://doi.org/10.1159/000499167
Nishida A, Andoh A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025;14(7).
https://doi.org/10.3390/cells14070488
Marcellinaro R, Spoletini D, Grieco M, Avella P, Cappuccio M, Troiano R, et al. Colorectal Cancer: Current Updates and Future Perspectives. J Clin Med 2023;13(1).
https://doi.org/10.3390/jcm13010040
Machala M, Procházková J, Hofmanová J, Králiková L, Slavík J, Tylichová Z, et al. Colon Cancer and Perturbations of the Sphingolipid Metabolism. Int J Mol Sci 2019;20(23).
https://doi.org/10.3390/ijms20236051
Zhakupova A, Zeinolla A, Kokabi K, Sergazy S, Aljofan M. Drug Resistance: The Role of Sphingolipid Metabolism. Int J Mol Sci 2025;26(8).
https://doi.org/10.3390/ijms26083716
Markowski AR, Błachnio-Zabielska AU, Pogodzińska K, Markowska AJ, Zabielski P. Diverse Sphingolipid Profiles in Rectal and Colon Cancer. Int J Mol Sci 2023;24(13).
https://doi.org/10.3390/ijms241310867
Alizadeh J, da Silva Rosa SC, Weng X, Jacobs J, Lorzadeh S, Ravandi A, et al. Ceramides and ceramide synthases in cancer: Focus on apoptosis and autophagy. Eur J Cell Biol 2023;102(3):151337.
https://doi.org/10.1016/j.ejcb.2023.151337
Vijayan Y, James S, Viswanathan A, Aparna JS, Bindu A, Namitha NN, et al. Targeting acid ceramidase enhances antitumor immune response in colorectal cancer. J Adv Res 2024;65:73-87.
https://doi.org/10.1016/j.jare.2023.12.013
Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol 2009;158(4):982-93.
https://doi.org/10.1111/j.1476-5381.2009.00281.x
Zeidan YH, Jenkins RW, Korman JB, Liu X, Obeid LM, Norris JS, et al. Molecular targeting of acid ceramidase: implications to cancer therapy. Curr Drug Targets 2008;9(8):653-61.
https://doi.org/10.2174/138945008785132358
Vijayan Y, Lankadasari MB, Harikumar KB. Acid Ceramidase: A Novel Therapeutic Target in Cancer. Curr Top Med Chem 2019;19(17):1512-20.
https://doi.org/10.2174/1568026619666190227222930
Bowden DL, Sutton PA, Wall MA, Jithesh PV, Jenkins RE, Palmer DH, et al. Proteomic profiling of rectal cancer reveals acid ceramidase is implicated in radiation response. J Proteomics 2018;179:53-60.
https://doi.org/10.1016/j.jprot.2018.02.030
Klobučar M, Grbčić P, Pavelić SK, Jonjić N, Visentin S, Sedić M. Acid ceramidase inhibition sensitizes human colon cancer cells to oxaliplatin through downregulation of transglutaminase 2 and β1 integrin/FAK-mediated signalling. Biochem Biophys Res Commun 2018;503(2):843-8.
https://doi.org/10.1016/j.bbrc.2018.06.085
Clifford RE, Govindarajah N, Bowden D, Sutton P, Glenn M, Darvish-Damavandi M, et al. Targeting Acid Ceramidase to Improve the Radiosensitivity of Rectal Cancer. Cells 2020;9(12).
https://doi.org/10.3390/cells9122693
Jang SW, Park WJ, Min H, Kwon TK, Baek SK, Hwang I, et al. Altered mRNA expression levels of the major components of sphingolipid metabolism, ceramide synthases and their clinical implication in colorectal cancer. Oncol Rep 2018;40(6):3489-500.
https://doi.org/10.3892/or.2018.6712
Espinoza KS, Snider AJ. Therapeutic Potential for Sphingolipids in Inflammatory Bowel Disease and Colorectal Cancer. Cancers (Basel) 2024;16(4).
https://doi.org/10.3390/cancers16040789
Markowski AR, Błachnio-Zabielska AU, Guzińska-Ustymowicz K, Markowska A, Pogodzińska K, Roszczyc K, et al. Ceramides Profile Identifies Patients with More Advanced Stages of Colorectal Cancer. Biomolecules 2020;10(4).
https://doi.org/10.3390/biom10040632
Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 2017;63:122-31.
https://doi.org/10.1016/j.jbior.2016.10.002
Leonetti D, Estéphan H, Ripoche N, Dubois N, Aguesse A, Gouard S, et al. Secretion of Acid Sphingomyelinase and Ceramide by Endothelial Cells Contributes to Radiation-Induced Intestinal Toxicity. Cancer Res 2020;80(12):2651-62.
https://doi.org/10.1158/0008-5472.CAN-19-1527
Scopa CD, Vagianos C, Kardamakis D, Kourelis TG, Kalofonos HP, Tsamandas AC. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with rectal cancer. Appl Immunohistochem Mol Morphol 2001;9(4):329-34.
https://doi.org/10.1097/00129039-200112000-00007
Bjelanović J, Nikolić A, Aslan M, Miladinov M, Kotur N, Barišić G, et al. Altered levels of sphingolipid metabolites in serum of locally advanced rectal cancer patients: A pilot study. J Med Biochem 2025;44(3):524-33.
https://doi.org/10.5937/jomb0-55113
Gabbani M, Giorgi C, Napoli G, Tebano U, Perrone MS, Missiroli S, et al. Outcomes of Locally Advanced Rectal Cancer Patients Treated with Total Neoadjuvant Treatment: A Meta-Anaysis of Randomized Controlled Trials. Clin Colorectal Cancer 2022;21(4):297-308.
https://doi.org/10.1016/j.clcc.2022.07.005
Yang M, Lin SQ, Liu XY, Tang M, Hu CL, Wang ZW, et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front Immunol 2023;14:1131496.
https://doi.org/10.3389/fimmu.2023.1131496
Furukawa S, Hiraki M, Kimura N, Kohya N, Sakai M, Ikubo A, et al. The Potential of the C-Reactive Protein-Albumin-Lymphocyte (CALLY) Index as a Prognostic Biomarker in Colorectal Cancer. Cancer Diagn Progn 2025;5(3):370-7.
https://doi.org/10.21873/cdp.10449
Wu B, Liu J, Shao C, Yu D, Liao J. Integrating inflammation, nutrition, and immunity: the CALLY index as a prognostic tool in digestive system cancers - a systematic review and meta-analysis. BMC Cancer 2025;25(1):672.
https://doi.org/10.1186/s12885-025-14074-3
Takeda Y, Sugano H, Okamoto A, Nakano T, Shimoyama Y, Takada N, et al. Prognostic usefulness of the C-reactive protein-albumin-lymphocyte (CALLY) index as a novel biomarker in patients undergoing colorectal cancer surgery. Asian J Surg 2024;47(8):3492-8.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Jasna Bjelanovic, Katarina Zeljic, Marko Miladinov, Goran Barisic, Sandra Dragicevic

This work is licensed under a Creative Commons Attribution 4.0 International License.









