Fecal DNA SDC2 methylation test for colorectal cancer diagnosis: A systematic review and meta-analysis
DOI:
https://doi.org/10.17305/bb.2026.13425Keywords:
Syndecan-2, DNA methylation, stool, colorectal cancer, diagnosisAbstract
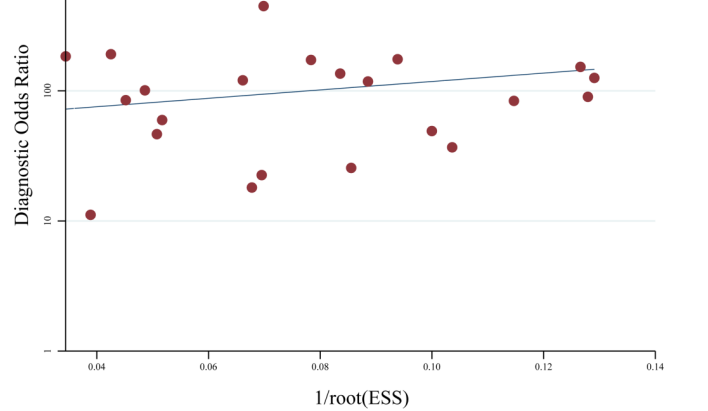
Fecal DNA methylation of the syndecan-2 (SDC2) gene is being explored as a noninvasive biomarker for colorectal cancer (CRC) detection. However, its diagnostic performance necessitates thorough evaluation. A systematic search of PubMed, Embase, and Web of Science was conducted to identify studies investigating fecal SDC2 methylation (mSDC2) for CRC diagnosis. Eligible studies included adult CRC patients with histological confirmation and controls with either normal mucosa or benign colorectal lesions. Pooled sensitivity and specificity were synthesized using a Reitsma bivariate random-effects model, and summary receiver operating characteristic (SROC) curves with corresponding area under the curve (AUC) values were derived from this hierarchical model. Twenty-five studies encompassing 3,427 CRC patients, 3,267 individuals with benign lesions, and 5,372 with normal mucosa were included. For the comparison of CRC versus normal mucosa (24 studies), the pooled sensitivity and specificity were 0.86 (95% confidence interval [CI]: 0.82-0.89; I² = 88%) and 0.93 (95% CI: 0.90-0.95; I² = 95%), respectively. The pooled diagnostic odds ratio (DOR) was 81.73 (95% CI: 51.60-129.46), with an AUC of 0.95 (95% CI: 0.93-0.97). In the comparison against benign lesions (22 studies), the sensitivity was 0.85 (95% CI: 0.81-0.89; I² = 87%), specificity was 0.66 (95% CI: 0.59-0.71; I² = 91%), DOR was 11.10 (95% CI: 7.61-16.19), and AUC was 0.83 (95% CI: 0.80-0.86). Deeks' funnel plot asymmetry tests indicated no statistically significant publication bias (p = 0.48 and 0.54). In conclusion, fecal mSDC2 testing demonstrates high diagnostic accuracy for CRC detection when compared to individuals with normal mucosa and moderate performance against benign colorectal lesions. These findings suggest that mSDC2 may serve as a promising noninvasive biomarker to complement existing CRC screening methodologies.
Citations
Downloads
References
Roshandel G, Ghasemi-Kebria F, Malekzadeh R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers. 2024;16(8):1530.
https://doi.org/10.3390/cancers16081530
Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10–45.
https://doi.org/10.3322/caac.21871
Mo S, Dai W, Wang H, Lan X, Ma C, Su Z, et al. Early detection and prognosis prediction for colorectal cancer by circulating tumour DNA methylation haplotypes: a multicentre cohort study. eClinicalMedicine. 2023;55:101717.
https://doi.org/10.1016/j.eclinm.2022.101717
Cardoso R, Guo F, Heisser T, De Schutter H, Van Damme N, Nilbert MC, et al. Overall and stage-specific survival of patients with screen-detected colorectal cancer in European countries: A population-based study in 9 countries. The Lancet Regional Health – Europe. 2022;21.
https://doi.org/10.1016/j.lanepe.2022.100458
Tsukanov VV, Vasyutin AV, Tonkikh JL. Risk factors, prevention and screening of colorectal cancer: A rising problem. World J Gastroenterol. 2025;31(5):98629.
https://doi.org/10.3748/wjg.v31.i5.98629
Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112(7):1016–30.
https://doi.org/10.1038/ajg.2017.174
Duan C, Sheng J, Ma X. Innovative approaches in colorectal cancer screening: advances in detection methods and the role of artificial intelligence. Ther Adv Gastroenterol. 2025;18:17562848251314829.
https://doi.org/10.1177/17562848251314829
Shaukat A, Levin TR. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. 2022;19(8):521–31.
https://doi.org/10.1038/s41575-022-00612-y
Mytilinaiou M, Nikitovic D, Berdiaki A, Kostouras A, Papoutsidakis A, Tsatsakis AM, et al. Emerging roles of syndecan 2 in epithelial and mesenchymal cancer progression. IUBMB Life. 2017;69(11):824–33.
https://doi.org/10.1002/iub.1678
Rezkitha YAA, Panenggak NSR, Lusida MI, Rianda RV, Mahmudah I, Pradana AD, et al. Detecting colorectal cancer using genetic and epigenetic biomarkers: screening and diagnosis. J Med Life. 2024;17(1):4–14.
https://doi.org/10.25122/jml-2023-0269
Kim JH, Park SC. Syndecan-2 Methylation as a New Biomarker for Early Detection of Colorectal Neoplasm. Gut Liver. 2018;12(5):479–80.
https://doi.org/10.5009/gnl18286
Fatemi N, Tierling S, Es HA, Varkiani M, Mojarad EN, Aghdaei HA, et al. DNA methylation biomarkers in colorectal cancer: Clinical applications for precision medicine. Int J Cancer. 2022;151(12):2068–81.
https://doi.org/10.1002/ijc.34186
Gómez-Molina R, Suárez M, Martínez R, Chilet M, Bauça JM, Mateo J. Utility of Stool-Based Tests for Colorectal Cancer Detection: A Comprehensive Review. Healthcare. 2024;12(16):1645.
https://doi.org/10.3390/healthcare12161645
Wang L, Liu Y, Zhang D, Xiong X, Hao T, Zhong L, et al. Diagnostic accuracy of DNA-based SDC2 methylation test in colorectal cancer screening: a meta-analysis. BMC Gastroenterol. 2022;22(1):314.
https://doi.org/10.1186/s12876-022-02395-7
Yue C, Zhang Y, Wang Y, Zhang Z, Zhang M, Wang H, et al. The Application Value of Syndecan-2 Gene Methylation for Colorectal Cancer Diagnosis: A Clinical Study and Meta-Analyses. Front Med (Lausanne). 2022;9:753545.
https://doi.org/10.3389/fmed.2022.753545
Cheng Z, Wang Y, Wei W, Liu Y, Zhou Z. Correlation between Stool DNA-based SDC2 Methylation Tests Combined with Tumor Markers with Pathological Features in Colorectal Cancer. Clin Lab. 2023;69(7).
https://doi.org/10.7754/Clin.Lab.2023.230303
Liu YM, Peng L, Chen C, Zhou P, Cheng B, Luo Y, et al. Value of faecal exfoliated cells in colorectal tumour screening using SDC2 methylation test. Ann Med. 2023;55(2):2261111.
https://doi.org/10.1080/07853890.2023.2261111
Xu J, Rong L, Gu F, You P, Ding H, Zhai H, et al. Asia-Pacific Colorectal Screening Score Combined With Stool DNA Test Improves the Detection Rate for Colorectal Advanced Neoplasms. Clin Gastroenterol Hepatol. 2023;21(6):1627–36.e4.
https://doi.org/10.1016/j.cgh.2022.09.002
Zeng T, Huang Z, Yu X, Zheng L, Liu T, Tian B, et al. Combining methylated SDC2 test in stool DNA, fecal immunochemical test, and tumor markers improves early detection of colorectal neoplasms. Front Oncol. 2023;13:1166796.
https://doi.org/10.3389/fonc.2023.1166796
Zhan Y, Wang S, Yuan Z, Zhao X, Ni K, Xin R, et al. The stool syndecan2 methylation test is more robust than blood tests for methylated septin9, CEA, CA19-9 and CA724: a diagnostic test for the early detection of colorectal neoplasms. Transl Cancer Res. 2023;12(1):65–77.
https://doi.org/10.21037/tcr-22-1710
Li N, Li C, Zhang X, Wang F, Li L, Liang J. Diagnostic value of human fecal SDC2 gene in colorectal cancer. Am J Transl Res. 2023a;15(4):2843–9.
Li B, Liu S, Gao Y, Zheng L, Lu Y. Combined detection of SDC2/ADHFE1/PPP2R5C methylation in stool DNA for colorectal cancer screening. J Cancer Res Clin Oncol. 2023b;149(12):10241–53.
https://doi.org/10.1007/s00432-023-04943-4
Kim CW, Kim H, Kim HR, Won DD, Nam WJ, Min BS, et al. A Stool DNA-Based SDC2 Methylation Test for the Early Detection of Colorectal Cancer in an Asymptomatic, High-Risk Population: A Multicenter Prospective Randomized Trial. Am J Gastroenterol. 2024;120(3):614–22.
https://doi.org/10.14309/ajg.0000000000003044
Liu Y, Ming H, Xu L, Li L, Liu Q, Zhao J, et al. DNA methylation analysis of the SDC2, SEPT9 and VIM genes in fecal DNA for colorectal cancer diagnosis. BMC Cancer. 2024;24(1):1205.
https://doi.org/10.1186/s12885-024-12990-4
Lohsiriwat V, Mongkhonsupphawan A, Ovartchaiyapong P. Diagnostic Accuracy of Multitarget Stool DNA Test for Colorectal Cancer Screening and Detecting in Thailand. Asian Pac J Cancer Prev. 2024;25(10):3661–5.
https://doi.org/10.31557/APJCP.2024.25.10.3661
Long L, Sun Q, Yang F, Zhou H, Wang Y, Xiao C, et al. Significance of SDC2 and NDRG4 methylation in stool for colorectal cancer diagnosis. Clin Biochem. 2024;124:110717.
https://doi.org/10.1016/j.clinbiochem.2024.110717
Luo WF, Jiao YT, Lin XL, Zhao Y, Wang SB, Shen J, et al. Effectiveness of fecal DNA syndecan-2 methylation testing for detection of colorectal cancer in a high-risk Chinese population. World J Gastrointest Oncol. 2024;16(4):1361–73.
https://doi.org/10.4251/wjgo.v16.i4.1361
Zhang K, He Q, Cao Q, Chuan J, Qin A, Tang L, et al. Evaluating the clinical performance of SDC2/NDRG4 methylation for colorectal cancer detection. Epigenomics. 2024;16(2):93–108.
https://doi.org/10.2217/epi-2023-0290
Zhao S, He Z, Sui X, Zhang S, Li Z, Bai Y. Real-World Stool-Based Syndecan-2 Methylation Test Improved Detection of Advanced Colorectal Neoplasia for Colorectal Cancer Screening: A Prospective, Multicenter, Community-Based Study. Gastroenterology. 2024;167(3):611–4.e7.
https://doi.org/10.1053/j.gastro.2024.04.019
Zou CS, Xie YL, Wang DX, Liu YP, Li MQ, Chen Y, et al. The value of SDC2 and Septin9 combined with serum tumor markers in early diagnosis of colorectal cancer. Int J Colorectal Dis. 2024;39(1):142.
https://doi.org/10.1007/s00384-024-04713-9
Liu X, Zhao G, Mao W, Li Q, Liao J, He G. Clinical significance of fecal Syndecan-2 gene methylation combined with blood tumor abnormal protein detection in the diagnosis of colorectal cancer and precancerous lesions. Clin Biochem. 2025;136:110887.
https://doi.org/10.1016/j.clinbiochem.2025.110887
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
https://doi.org/10.1136/bmj.n71
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
https://doi.org/10.1136/bmj.n160
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. 2021.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35.
https://doi.org/10.1016/S0895-4356(03)00177-X
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
https://doi.org/10.1002/sim.1186
van Enst WA, Ochodo E, Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14:70.
https://doi.org/10.1186/1471-2288-14-70
Niu F, Wen J, Fu X, Li C, Zhao R, Wu S, et al. Stool DNA Test of Methylated Syndecan-2 for the Early Detection of Colorectal Neoplasia. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1411–9.
https://doi.org/10.1158/1055-9965.EPI-17-0153
Oh TJ, Oh HI, Seo YY, Jeong D, Kim C, Kang HW, et al. Feasibility of quantifying SDC2 methylation in stool DNA for early detection of colorectal cancer. Clin Epigenetics. 2017;9:126.
https://doi.org/10.1186/s13148-017-0426-3
Han YD, Oh TJ, Chung TH, Jang HW, Kim YN, An S, et al. Early detection of colorectal cancer based on presence of methylated syndecan-2 (SDC2) in stool DNA. Clin Epigenetics. 2019;11(1):51.
https://doi.org/10.1186/s13148-019-0642-0
Sun M, Liu J, Hu H, Guo P, Shan Z, Yang H, et al. A novel panel of stool-based DNA biomarkers for early screening of colorectal neoplasms in a Chinese population. J Cancer Res Clin Oncol. 2019;145(10):2423–32.
https://doi.org/10.1007/s00432-019-02992-2
Wang J, Liu S, Wang H, Zheng L, Zhou C, Li G, et al. Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: a multicenter clinical study. Clin Epigenetics. 2020;12(1):162.
https://doi.org/10.1186/s13148-020-00954-x
Su WC, Kao WY, Chang TK, Tsai HL, Huang CW, Chen YC, et al. Stool DNA test targeting methylated syndecan-2 (SDC2) as a noninvasive screening method for colorectal cancer. Biosci Rep. 2021;41(1).
https://doi.org/10.1042/BSR20201930
Zhang W, Yang C, Wang S, Xiang Z, Dou R, Lin Z, et al. SDC2 and TFPI2 Methylation in Stool Samples as an Integrated Biomarker for Early Detection of Colorectal Cancer. Cancer Manag Res. 2021;13:3601–17.
https://doi.org/10.2147/CMAR.S300861
Dai Y, Zhao G, Yang J, Zhou X, Xiong S, Lu X, et al. A simplified multiplex methylated DNA testing for early detection of colorectal cancer in stool DNA. BMC Gastroenterol. 2022;22(1):428.
https://doi.org/10.1186/s12876-022-02512-6
Ma L, Qin G, Gai F, Jiang Y, Huang Z, Yang H, et al. A novel method for early detection of colorectal cancer based on detection of methylation of two fragments of syndecan-2 (SDC2) in stool DNA. BMC Gastroenterol. 2022;22(1):191.
https://doi.org/10.1186/s12876-022-02264-3
Lee H, Kim Y, Choi Y, Choi S, Hong E, Oh ES. Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem Biophys Res Commun. 2011;409(1):148–53.
https://doi.org/10.1016/j.bbrc.2011.04.135
Vicente CM, da Silva DA, Sartorio PV, Silva TD, Saad SS, Nader HB, et al. Heparan Sulfate Proteoglycans in Human Colorectal Cancer. Anal Cell Pathol (Amst). 2018;2018:8389595.
https://doi.org/10.1155/2018/8389595
Rodriguez-Casanova A, Costa-Fraga N, Bao-Caamano A, López-López R, Muinelo-Romay L, Diaz-Lagares A. Epigenetic Landscape of Liquid Biopsy in Colorectal Cancer. Front Cell Dev Biol. 2021;9:622459.
https://doi.org/10.3389/fcell.2021.622459
Borinstein SC, Conerly M, Dzieciatkowski S, Biswas S, Washington MK, Trobridge P, et al. Aberrant DNA methylation occurs in colon neoplasms arising in the azoxymethane colon cancer model. Mol Carcinog. 2010;49(1):94–103.
https://doi.org/10.1002/mc.20581
Nagasaka T, Tanaka N, Cullings HM, Sun DS, Sasamoto H, Uchida T, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101(18):1244–58.
https://doi.org/10.1093/jnci/djp265
Kong X, Wu Q, Zhang Z, Yu Z, Niu F, Wang X, et al. Effectiveness of single-target fecal DNA methylation test in regional mass screening for colorectal cancer and precancerous lesions in China. Gastroenterol Rep (Oxf). 2025;13:goaf029.
https://doi.org/10.1093/gastro/goaf029
Porcaro F, Voccola S, Cardinale G, Porcaro P, Vito P. DNA Methylation Biomarkers in Stool Samples: Enhancing Colorectal Cancer Screening Strategies. Oncol Rev. 2024;18:1408529.
https://doi.org/10.3389/or.2024.1408529
Stonestreet J, Chandrapalan S, Woolley D, Uthman U, Arasaradnam RP. Systematic review and meta-analysis : diagnostic accuracy of faecal immunochemical testing for haemoglobin (FIT) in detecting colorectal cancer for both symptomatic and screening population. Acta Gastroenterol Belg. 2019;82(2):291–9.
Dolatkhah R, Dastgiri S, Jafarabadi MA, Abdolahi HM, Somi MH. Diagnostic accuracy of multitarget stool DNA testing for colorectal cancer screening: A systematic review and meta-analysis. Gastroenterol Hepatol. 2022;45(10):753–66.
https://doi.org/10.1016/j.gastrohep.2022.01.007
Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 2018;6(2).
https://doi.org/10.3390/medsci6020031
Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–42.
https://doi.org/10.1097/PRS.0b013e3181f44abc
Partyka O, Pajewska M, Czerw A, Deptała A, Mękal D, Sygit K, et al. Current Progress in Clinical Research in Secondary Prevention and Early Detection of Colorectal Cancer. Cancers (Basel). 2025;17(3).
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2026 Xinxin Liu, Bing Yang, Dongxin Tang

This work is licensed under a Creative Commons Attribution 4.0 International License.









