Second-generation antipsychotics – Cardiac ion channel modulation and QT interval disturbances: A review
DOI:
https://doi.org/10.17305/bb.2025.13405Keywords:
Second-generation antipsychotics, QT prolongation, torsades de pointes, hERG potassium channels, sodium channels, calcium channels, repolarization reserve, ventricular arrhythmiasAbstract
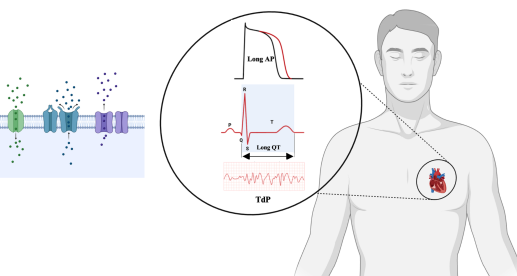
Second-generation antipsychotics (SGAs) are frequently prescribed in psychiatry due to their efficacy and improved tolerability compared to first-generation agents. However, these medications are associated with significant cardiac adverse effects, particularly QT interval prolongation and torsades de pointes (TdP). This review aims to summarize the mechanisms by which SGAs affect cardiac ion channels and how these actions contribute to QT interval disturbances and increased arrhythmia risk. A narrative literature review was conducted using PubMed, Web of Science, and Google Scholar, without year restrictions, focusing on English-language experimental and clinical studies related to clozapine, olanzapine, risperidone, quetiapine, and ziprasidone. The findings indicate that all five SGAs inhibit the rapid delayed rectifier potassium current (IKr) mediated by the human ether-a-go-go-related gene (hERG) potassium channel. Notably, the observed variability in the ratio of half-maximal inhibitory concentration to maximum free plasma concentration (IC₅₀/Cmax,free) reflects its dependence on both the degree of hERG inhibition and the pharmacokinetic properties specific to each SGA. Additionally, several SGAs affect other potassium, sodium, and calcium currents, which may either mitigate or exacerbate the consequences of IKr inhibition. In conclusion, QT interval prolongation associated with SGAs is primarily driven by hERG potassium channel blockade, although the degree of this effect varies significantly among different agents. This variability highlights the necessity for electrocardiogram (ECG) monitoring and individualized cardiac risk assessments, especially for vulnerable patient populations.
Citations
Downloads
References
Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World psychiatry. 2015;14(2):119–36.
https://doi.org/10.1002/wps.20204
Hudepohl NS, Nasrallah HA. Antipsychotic drugs. Handbook of Clinical Neurology. 2012;106:657–67.
https://doi.org/10.1016/B978-0-444-52002-9.00039-5
Fabrazzo M, Cipolla S, Camerlengo A, Perris F, Catapano F. Second-generation antipsychotics' effectiveness and tolerability: a review of real-world studies in patients with schizophrenia and related disorders. Journal of Clinical Medicine. 2022;11(15):4530.
https://doi.org/10.3390/jcm11154530
Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172(13):1703–11.
https://doi.org/10.1503/cmaj.1041064
Meltzer H, Matsubara S, Lee J. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacology bulletin. 1989;25(3):390–2.
Dayalu P, Chou KL. Antipsychotic-induced extrapyramidal symptoms and their management. Expert opinion on pharmacotherapy. 2008;9(9):1451–62.
https://doi.org/10.1517/14656566.9.9.1451
Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(7):1081–90.
https://doi.org/10.1016/j.pnpbp.2003.09.004
Miyamoto S, Miyake N, Jarskog L, Fleischhacker W, Lieberman J. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Molecular psychiatry. 2012;17(12):1206–27.
https://doi.org/10.1038/mp.2012.47
Serretti A, Ronchi DD, Lorenzi C, Berardi D. New antipsychotics and schizophrenia: a review on efficacy and side effects. Current medicinal chemistry. 2004;11(3):343–58.
https://doi.org/10.2174/0929867043456043
Stahl SM. Stahl's essential psychopharmacology: neuroscientific basis and practical applications. Cambridge University Press. 2021.
https://doi.org/10.1017/9781108975292
Meltzer HY, Fibiger HC. Olanzapine: a new atypical antipsychotic drug. Neuropsychopharmacology. 1996;14(2):83–5.
https://doi.org/10.1016/0893-133X(95)00197-L
Schrader JM, Irving CM, Octeau JC, Christian JA, Aballo TJ, Kareemo DJ, et al. The differential actions of clozapine and other antipsychotic drugs on the translocation of dopamine D2 receptors to the cell surface. Journal of Biological Chemistry. 2019;294(14):5604–15.
https://doi.org/10.1074/jbc.RA118.004682
Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: A new hypothesis. American Journal of Psychiatry. 2001;158(3):360–9.
https://doi.org/10.1176/appi.ajp.158.3.360
Meltzer HY. Update on typical and atypical antipsychotic drugs. Annual review of medicine. 2013;64(1):393–406.
https://doi.org/10.1146/annurev-med-050911-161504
Conley RR. Risperidone side effects. Journal of Clinical Psychiatry. 2000;61(4):20–5.
Khokhar JY, Henricks AM, Sullivan ED, Green AI. Unique effects of clozapine: a pharmacological perspective. Advances in pharmacology. 2018;82:137–62.
https://doi.org/10.1016/bs.apha.2017.09.009
McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophrenia Research. 2010;122(1–3):151–5.
https://doi.org/10.1016/j.schres.2010.03.034
Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. The Lancet. 2013;382(9896):951–62.
https://doi.org/10.1016/S0140-6736(13)60733-3
Carli M, Kolachalam S, Longoni B, Pintaudi A, Baldini M, Aringhieri S, et al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals. 2021;14(3):238.
https://doi.org/10.3390/ph14030238
Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. New England Journal of Medicine. 2009;360(3):225–35.
https://doi.org/10.1056/NEJMoa0806994
Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Archives of General Psychiatry. 2001;58(12):1161–7.
https://doi.org/10.1001/archpsyc.58.12.1161
Sicouri S, Antzelevitch C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert opinion on drug safety. 2008;7(2):181–94.
https://doi.org/10.1517/14740338.7.2.181
Wu CS, Tsai YT, Tsai HJ. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation‐wide case‐crossover study. Journal of the American Heart Association. 2015;4(2):e001568.
https://doi.org/10.1161/JAHA.114.001568
Christ T, Wettwer E, Ravens U. Risperidone-induced action potential prolongation is attenuated by increased repolarization reserve due to concomitant block of ICa,L. Naunyn-Schmiedeberg's Archives of Pharmacology. 2005;371(5):393–400.
https://doi.org/10.1007/s00210-005-1063-5
Crumb Jr WJ, Ekins S, Sarazan RD, Wikel JH, Wrighton SA, Carlson C, et al. Effects of antipsychotic drugs on Ito, INa, Isus, IK1, and hERG: QT prolongation, structure activity relationship, and network analysis. Pharmaceutical Research. 2006;23(6):1133–43.
https://doi.org/10.1007/s11095-006-0070-7
Gluais P, Bastide M, Grandmougin D, Fayad G, Adamantidis M. Risperidone reduces K+ currents in human atrial myocytes and prolongs repolarization in human myocardium. European Journal of Pharmacology. 2004;497(2):215–22.
https://doi.org/10.1016/j.ejphar.2004.06.046
De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues in Clinical Neuroscience. 2018;20(1):31–40.
https://doi.org/10.31887/DCNS.2018.20.1/mdehert
Brown CS, Farmer RG, Soberman JE, Eichner SF. Pharmacokinetic factors in the adverse cardiovascular effects of antipsychotic drugs. Clinical Pharmacokinetics. 2004;43(1):33–56.
https://doi.org/10.2165/00003088-200443010-00003
Rødevand L, Rahman Z, Hindley GF, Smeland OB, Frei O, Tekin TF, et al. Characterizing the shared genetic underpinnings of schizophrenia and cardiovascular disease risk factors. American Journal of Psychiatry. 2023;180(11):815–26.
https://doi.org/10.1176/appi.ajp.20220660
Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Safety. 2000;23(3):215–28.
https://doi.org/10.2165/00002018-200023030-00004
Kilicaslan EE, Karakilic M, Erol A. The relationship between 10 years risk of cardiovascular disease and schizophrenia symptoms: preliminary results. Psychiatry Investigation. 2019;16(12):933.
https://doi.org/10.30773/pi.2019.0063
Gugger JJ. Antipsychotic pharmacotherapy and orthostatic hypotension: identification and management. CNS Drugs. 2011;25(8):659–71.
https://doi.org/10.2165/11591710-000000000-00000
Liperoti R, Gambassi G, Lapane KL, Chiang C, Pedone C, Mor V, et al. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Archives of Internal Medicine. 2005;165(6):696–701.
https://doi.org/10.1001/archinte.165.6.696
Ruiz Diaz JC, Frenkel D, Aronow WS. The relationship between atypical antipsychotics drugs, QT interval prolongation, and torsades de pointes: implications for clinical use. Expert Opinion on Drug Safety. 2020;19(5):559–64.
https://doi.org/10.1080/14740338.2020.1745184
Salvo F, Pariente A, Shakir S, Robinson P, Arnaud M, Thomas S, et al. Sudden cardiac and sudden unexpected death related to antipsychotics: a meta‐analysis of observational studies. Clinical Pharmacology & Therapeutics. 2016;99(3):306–14.
https://doi.org/10.1002/cpt.250
Vieweg WVR. New generation antipsychotic drugs and QTc interval prolongation. Primary Care Companion to The Journal of Clinical Psychiatry. 2003;5(5):205.
https://doi.org/10.4088/PCC.v05n0504
Bellissima BL, Tingle MD, Cicović A, Alawami M, Kenedi C. A systematic review of clozapine-induced myocarditis. International Journal of Cardiology. 2018;259:122–9.
https://doi.org/10.1016/j.ijcard.2017.12.102
Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
https://doi.org/10.1016/j.psym.2012.11.001
Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307.
https://doi.org/10.1016/0092-8674(95)90340-2
Thomas SH, Behr ER. Pharmacological treatment of acquired QT prolongation and torsades de pointes. British Journal of Clinical Pharmacology. 2016;81(3):420–7.
https://doi.org/10.1111/bcp.12726
Moss A, Schwartz P, Crampton R, Tzivoni D, Locati E, MacCluer J, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84(3):1136–44.
https://doi.org/10.1161/01.CIR.84.3.1136
Moss AJ. The QT interval and torsade de pointes. Drug Safety. 1999;21(Suppl 1):5–10.
https://doi.org/10.2165/00002018-199921001-00002
Naheed M, Green B. Focus on clozapine. Current Medical Research and Opinion. 2001;17(3):223–9.
https://doi.org/10.1185/03007990152673864
Alvir JMJ, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis--incidence and risk factors in the United States. New England Journal of Medicine. 1993;329(3):162–7.
https://doi.org/10.1056/NEJM199307153290303
Chapelle A, Kari C, Nurminen M, Hernberg S. Clozapine-induced agranulocytosis. Human Genetics. 1977;37(2):183–94.
https://doi.org/10.1007/BF00393581
Schulte PF. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Annals of Pharmacotherapy. 2006;40(4):683–8.
https://doi.org/10.1345/aph.1G396
Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proceedings of the National Academy of Sciences. 2003;100(23):13674–9.
https://doi.org/10.1073/pnas.1835612100
Sankaranarayanan A, Kazi S, Andrade C. Prevalence and predictors of QTc prolongation in patients seen in a clozapine clinic in a real-world setting in Australia. Schizophrenia Research. 2024;268:145–9.
https://doi.org/10.1016/j.schres.2023.09.032
Lee SY, Kim YJ, Kim KT, Choe H, Jo SH. Blockade of HERG human K+ channels and IKr of guinea‐pig cardiomyocytes by the antipsychotic drug clozapine. British Journal of Pharmacology. 2006;148(4):499–509.
https://doi.org/10.1038/sj.bjp.0706744
Hill AP, Perrin MJ, Heide J, Campbell TJ, Mann SA, Vandenberg JI. Kinetics of drug interaction with the Kv11.1 potassium channel. Molecular Pharmacology. 2014;85(5):769–76.
https://doi.org/10.1124/mol.114.091835
Le Marois M, Sanson C, Maizières M-A, Partiseti M, Bohme GA. The atypic antipsychotic clozapine inhibits multiple cardiac ion channels. Naunyn-Schmiedeberg's Archives of Pharmacology. 2023;396(1):161–6.
https://doi.org/10.1007/s00210-022-02314-3
Kang M, Heo R, Park S, Mun S-Y, Park M, Han E-T, et al. Inhibitory effects of the atypical antipsychotic, clozapine, on voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2022;26(4):277–85.
https://doi.org/10.4196/kjpp.2022.26.4.277
Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. The Journal of Pharmacology and Experimental Therapeutics. 1998;286(2):709–17.
https://doi.org/10.1016/S0022-3565(24)37644-X
Choi K-H, Rhim H. Inhibition of recombinant Cav3.1 (α1G) T-type calcium channels by the antipsychotic drug clozapine. European Journal of Pharmacology. 2010;626(2–3):123–30.
https://doi.org/10.1016/j.ejphar.2009.09.035
Broich K, Heinrich S, Marneros A. Acute clozapine overdose: plasma concentration and outcome. Pharmacopsychiatry. 1998;31(04):149–51.
https://doi.org/10.1055/s-2007-979318
Chang W, Lin S, Lane H, Hu W, Jann M, Lin H. Clozapine dosages and plasma drug concentrations. Journal of the Formosan Medical Association = Taiwan yi zhi. 1997;96(8):599–605.
Gründer G, Landvogt C, Vernaleken I, Buchholz H-G, Ondracek J, Siessmeier T, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027–35.
https://doi.org/10.1038/sj.npp.1300931
Hiemke C, Bergemann N, Clement H, Conca A, Deckert J, Domschke K, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(01/02):9–62.
https://doi.org/10.1055/s-0043-116492
Perry PJ, Miller DD, Arndt SV, Cadoret RJ. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. The American Journal of Psychiatry. 1991;148(2):231–5.
https://doi.org/10.1176/ajp.148.2.231
Arzuk E, Karakuş F, Orhan H. Bioactivation of clozapine by mitochondria of the murine heart: Possible cause of cardiotoxicity. Toxicology. 2021;447:152628.
https://doi.org/10.1016/j.tox.2020.152628
Titier K, Canal M, Déridet E, Abouelfath A, Gromb S, Molimard M, et al. Determination of myocardium to plasma concentration ratios of five antipsychotic drugs: comparison with their ability to induce arrhythmia and sudden death in clinical practice. Toxicology and Applied Pharmacology. 2004;199(1):52–60.
https://doi.org/10.1016/j.taap.2004.03.016
Warner B, Hoffmann P. Investigation of the potential of clozapine to cause torsade de pointes. Adverse Drug Reactions and Toxicological Reviews. 2002;21(4):189–203.
https://doi.org/10.1007/BF03256196
Moore NA, Tye NC, Axton MS, Risius FC. The behavioral pharmacology of olanzapine, a novel "atypical" antipsychotic agent. The Journal of Pharmacology and Experimental Therapeutics. 1992;262(2):545–51.
https://doi.org/10.1016/S0022-3565(25)10792-1
Thomas K, Saadabadi A. Olanzapine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532903/
Adel N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. The American Journal of Managed Care. 2017;23(14 Suppl):S259–S65.
Attia E, Steinglass JE, Walsh BT, Wang Y, Wu P, Schreyer C, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. American Journal of Psychiatry. 2019;176(6):449–56.
https://doi.org/10.1176/appi.ajp.2018.18101125
Markowitz JD, Narasimhan M. Delirium and antipsychotics: a systematic review of epidemiology and somatic treatment options. Psychiatry (Edgmont). 2008;5(10):29.
Wilson MP, Pepper D, Currier GW, Holloman Jr GH, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. Western Journal of Emergency Medicine. 2012;13(1):26.
https://doi.org/10.5811/westjem.2011.9.6866
Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14(2):87–96.
https://doi.org/10.1016/0893-133X(94)00129-N
Fuller R, Snoddy H. Neuroendocrine evidence for antagonism of serotonin and dopamine receptors by olanzapine (LY170053), an antipsychotic drug candidate. Research Communications in Chemical Pathology and Pharmacology. 1992;77(1):87–93.
Leung JY, Pang CC, Procyshyn RM, Barr AM. Cardiovascular effects of acute treatment with the antipsychotic drug olanzapine in rats. Vascular Pharmacology. 2014;62(3):143–9.
https://doi.org/10.1016/j.vph.2014.06.003
Pu Z-P, Li G-R, Zou Z-P, Tao F, Hu S-H. A randomized, 8-week study of the effects of extended-release paliperidone and olanzapine on heart rate variability in patients with schizophrenia. Journal of Clinical Psychopharmacology. 2019;39(3):243–8.
https://doi.org/10.1097/JCP.0000000000001023
Tajiri M, Suzuki Y, Sugai T, Tsuneyama N, Someya T. Effects of olanzapine on resting heart rate in Japanese patients with schizophrenia. PLoS One. 2018;13(7):e0199922.
https://doi.org/10.1371/journal.pone.0199922
Sun L, Yagoda S, Xue H, Brown R, Nangia N, McDonnell D, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2020;100:109881.
https://doi.org/10.1016/j.pnpbp.2020.109881
Alda JA, Munoz-Samons D, Tor J, Merchan-Naranjo J, Tapia-Casellas C, Baeza I, et al. Absence of change in corrected QT interval in children and adolescents receiving antipsychotic treatment: a 12 month study. Journal of Child and Adolescent Psychopharmacology. 2016;26(5):449–57.
https://doi.org/10.1089/cap.2015.0151
Ando K, Sugiyama A, Takahara A, Satoh Y, Ishizaka T, Nakamura Y, et al. Analysis of proarrhythmic potential of antipsychotics risperidone and olanzapine in anesthetized dogs. European Journal of Pharmacology. 2007;558(1–3):151–8.
https://doi.org/10.1016/j.ejphar.2006.11.078
Rivas JC, Galindo-A J, Zambrano LF, Miranda-B CA, Ramírez SM, Rivas-Grajales AM, et al. Risk of corrected QT interval prolongation in patients receiving antipsychotics. International Clinical Psychopharmacology. 2025;40(4):207–13.
https://doi.org/10.1097/YIC.0000000000000564
Lee HJ, Choi J-S, Hahn SJ. Mechanism of inhibition by olanzapine of cloned hERG potassium channels. Neuroscience Letters. 2015;609:97–102.
https://doi.org/10.1016/j.neulet.2015.10.039
Bergemann N, Frick A, Parzer P, Kopitz J. Olanzapine plasma concentration, average daily dose, and interaction with co-medication in schizophrenic patients. Pharmacopsychiatry. 2004;37(02):63–8.
https://doi.org/10.1055/s-2004-815527
Morissette P, Hreiche R, Mallet L, Vo D, Knaus EE, Turgeon J. Olanzapine prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current. Journal of Psychopharmacology. 2007;21(7):735–41.
https://doi.org/10.1177/0269881106072669
Ekins S, Crumb WJ, Sarazan RD, Wikel JH, Wrighton SA. Three-dimensional quantitative structure-activity relationship for inhibition of human ether-a-go-go-related gene potassium channel. The Journal of Pharmacology and Experimental Therapeutics. 2002;301(2):427–34.
https://doi.org/10.1124/jpet.301.2.427
Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. Journal of Clinical Psychopharmacology. 2004;24(1):62–9.
https://doi.org/10.1097/01.jcp.0000104913.75206.62
Mow T, Frederiksen K, Thomsen MB. Assessment of anti-arrhythmic activity of antipsychotic drugs in an animal model: Influence of non-cardiac α1-adrenergic receptors. European Journal of Pharmacology. 2015;748:10–7.
https://doi.org/10.1016/j.ejphar.2014.12.012
Lehmann DF, Eggleston WD, Wang D. Validation and clinical utility of the hERG IC50:Cmax ratio to determine the risk of drug‐induced torsades de pointes: A meta‐analysis. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2018;38(3):341–8.
https://doi.org/10.1002/phar.2087
Czekalla J, Beasley CM, Dellva MA, Berg PH, Grundy S. Analysis of the QTc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry. 2001;62(3):191–8.
https://doi.org/10.4088/JCP.v62n0310
Shoja Shafti S, Fallah Jahromi P. Olanzapine induced Q-Tc shortening. Therapeutic Advances in Psychopharmacology. 2014;4(6):240–6.
https://doi.org/10.1177/2045125314546484
Janssen PA, Niemegeers C, Awouters F, Schellekens K, Megens A, Meert T. Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. The Journal of Pharmacology and Experimental Therapeutics. 1988;244(2):685–93.
https://doi.org/10.1016/S0022-3565(25)24408-1
Leysen J, Gommeren W, Eens A, De Courcelles DDC, Stoof J, Janssen P. Biochemical profile of risperidone, a new antipsychotic. The Journal of Pharmacology and Experimental Therapeutics. 1988;247(2):661–70.
https://doi.org/10.1016/S0022-3565(25)13416-2
Bruggeman R, van der Linden C, Buitelaar JK, Gericke GS, Hawkridge SM, Temlett JA. Risperidone versus pimozide in Tourette's disorder: a comparative double-blind parallel-group study. Journal of Clinical Psychiatry. 2001;62(1):50–6.
https://doi.org/10.4088/JCP.v62n0111
Foster RH, Goa KL. Risperidone: a pharmacoeconomic review of its use in schizophrenia. Pharmacoeconomics. 1998;14(1):97–133.
https://doi.org/10.2165/00019053-199814010-00009
Galizia G. The treatment of the schizophrenia: an overview. Pharmacologyonline. 2009;2:971–1020.
Acri AA, Henretig FM. Effects of risperidone in overdose. The American Journal of Emergency Medicine. 1998;16(5):498–501.
https://doi.org/10.1016/S0735-6757(98)90001-8
Mazhar MN, Resch DS. Risperidone induced ventricular tachycardia. Psychopharmacology Bulletin. 2010;43(3):82–3.
https://doi.org/10.64719/pb.4298
Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. The Journal of Emergency Medicine. 2017;53(5):735–9.
https://doi.org/10.1016/j.jemermed.2017.06.026
Timour Q, Frassati D, Descotes J, Chevalier P, Christé G, Chahine M. Sudden death of cardiac origin and psychotropic drugs. Frontiers in Pharmacology. 2012;3:76.
https://doi.org/10.3389/fphar.2012.00076
Beach SR, Celano CM, Sugrue AM, Adams C, Ackerman MJ, Noseworthy PA, et al. QT prolongation, torsades de pointes, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(2):105–22.
https://doi.org/10.1016/j.psym.2017.10.009
Raviña T, Raviína P, Gutierrez J. Acquired long QT syndrome: Risperidone-facilitated triggered activity and torsades de pointes during complete AV block. I. International Journal of Cardiology. 2007;116(3):416–20.
https://doi.org/10.1016/j.ijcard.2006.04.084
Saha A, Kumar A, Singh S, Somani A. QT interval prolongation from antipsychotics in schizophrenia and acute psychosis–A prospective study. Industrial Psychiatry Journal. 2025;34(2):228–35.
https://doi.org/10.4103/ipj.ipj_495_24
Drolet B, Yang T, Daleau P, Roden DM, Turgeon J. Risperidone prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current. Journal of Cardiovascular Pharmacology. 2003;41(6):934–7.
https://doi.org/10.1097/00005344-200306000-00016
Fossa AA, Wisialowski T, Wolfgang E, Wang E, Avery M, Raunig DL, et al. Differential effect of HERG blocking agents on cardiac electrical alternans in the guinea pig. European Journal of Pharmacology. 2004;486(2):209–21.
https://doi.org/10.1016/j.ejphar.2003.12.028
Gluais P, Bastide M, Caron J, Adamantidis M. Risperidone prolongs cardiac action potential through reduction of K+ currents in rabbit myocytes. European Journal of Pharmacology. 2002;444(3):123–32.
https://doi.org/10.1016/S0014-2999(02)01626-6
Kobayashi T, Washiyama K, Ikeda K. Modulators of G protein‐activated inwardly rectifying K+ channels: potentially therapeutic agents for addictive drug users. Annals of the New York Academy of Sciences. 2004;1025(1):590–4.
https://doi.org/10.1196/annals.1316.073
Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Current Drug Safety. 2010;5(1):97–104.
https://doi.org/10.2174/157488610789869265
Raviña T, Gutierrez J, Raviña P. Acquired long QT syndrome: long-term electrocardiographic (Holter) recording of torsades de pointes ending in asystole: II. International Journal of Cardiology. 2007;116(2):272–5.
https://doi.org/10.1016/j.ijcard.2006.04.069
Vieweg WVR, Hasnain M, Hancox JC, Baranchuk A, Digby GC, Kogut C, et al. Risperidone, QTc interval prolongation, and torsade de pointes: a systematic review of case reports. Psychopharmacology. 2013;228(4):515–24.
https://doi.org/10.1007/s00213-013-3192-8
Kreifels P, Bodi I, Hornyik T, Franke G, Perez-Feliz S, Lewetag R, et al. Oxytocin exerts harmful cardiac repolarization prolonging effects in drug-induced LQTS. IJC Heart & Vasculature. 2022;40:101001.
https://doi.org/10.1016/j.ijcha.2022.101001
Magyar J, Bányász T, Bagi Z, Pacher P, Szentandrássy N, Fülöp L, et al. Electrophysiological effects of risperidone in mammalian cardiac cells. Naunyn-Schmiedeberg's Archives of Pharmacology. 2002;366(4):350–6.
https://doi.org/10.1007/s00210-002-0595-1
Brett J, Gillies MB, Buckley NA, Pearson SA, Zoega H. Patterns of suboptimal antipsychotic use and misuse in Australia: What can routinely collected data tell us? British Journal of Clinical Pharmacology. 2023;89(11):3411–20.
https://doi.org/10.1111/bcp.15821
Hawkins SB, Bucklin M, Muzyk AJ. Quetiapine for the treatment of delirium. Journal of Hospital Medicine. 2013;8(4):215–20.
https://doi.org/10.1002/jhm.2019
Sanford M. Quetiapine extended release. CNS Drugs. 2011;25(9):803–13.
https://doi.org/10.2165/11207280-000000000-00000
Strawbridge R, Kurana S, Kerr‐Gaffney J, Jauhar S, Kaufman KR, Yalin N, et al. A systematic review and meta‐analysis of treatments for rapid cycling bipolar disorder. Acta Psychiatrica Scandinavica. 2022;146(4):290–311.
https://doi.org/10.1111/acps.13471
Plosker GL. Quetiapine. Pharmacoeconomics. 2012;30(7):611–31.
https://doi.org/10.2165/11208500-000000000-00000
Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, De Gaspari IF, Bareggi SR. Clinical pharmacokinetics of atypical antipsychotics. Clinical Pharmacokinetics. 2007;46(5):359–88.
https://doi.org/10.2165/00003088-200746050-00001
Nemeroff CB, Kinkead B, Goldstein J. Quetiapine: preclinical studies, pharmacokinetics, drug interactions, and dosing. Journal of Clinical Psychiatry. 2002;63(13):5–11.
Casey D. "Seroque" (quetiapine): Preclinical and clinical findings of a new atypical antipsychotic. Expert Opinion on Investigational Drugs. 1996;5(8):939–57.
https://doi.org/10.1517/13543784.5.8.939
Saller CF, Salama AI. Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112(2):285–92.
https://doi.org/10.1007/BF02244923
Gefvert O, Lundberg T, Wieselgren I-M, Bergström M, Långström B, Wiesel F-A, et al. D2 and 5HT2A receptor occupancy of different doses of quetiapine in schizophrenia: a PET study. European Neuropsychopharmacology. 2001;11(2):105–10.
https://doi.org/10.1016/S0924-977X(00)00133-4
Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors: focus on newer generation compounds. Life Sciences. 2000;68(1):29–39.
https://doi.org/10.1016/S0024-3205(00)00911-5
Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsychopharmacology. 2008;33(10):2303–12.
https://doi.org/10.1038/sj.npp.1301646
Winter HR, Earley WR, Hamer-Maansson JE, Davis PC, Smith MA. Steady-state pharmacokinetic, safety, and tolerability profiles of quetiapine, norquetiapine, and other quetiapine metabolites in pediatric and adult patients with psychotic disorders. Journal of Child and Adolescent Psychopharmacology. 2008;18(1):81–98.
https://doi.org/10.1089/cap.2007.0084
Nyberg S, Takano A, Grimm S, Gulyas B, McCarthy D, Lee C, et al. P.1.c.022 PET-measured D2, 5-HT2, and norepinephrine transporter (NET) occupancy by quetiapine and N-desalkyl-quetiapine in non-human primates. European Neuropsychopharmacology. 2007;17(Suppl):S254–S5.
https://doi.org/10.1016/S0924-977X(07)70346-2
Prieto E, Micó J, Meana J, Majadas S. Neurobiological bases of quetiapine antidepressant effect in the bipolar disorder. Actas Esp Psiquiatr. 2010;38(1):22–32.
Bergman H, Walker D-M, Nikolakopoulou A, Soares-Weiser K, Adams CE. Systematic review of interventions for treating or preventing antipsychotic-induced tardive dyskinesia. Health Technology Assessment. 2017;21(43):1–218.
https://doi.org/10.3310/hta21430
Caccia S, Clavenna A, Bonati M. Antipsychotic drug toxicology in children. Expert Opinion on Drug Metabolism & Toxicology. 2011;7(5):591–608.
https://doi.org/10.1517/17425255.2011.562198
Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Therapeutics and Clinical Risk Management. 2017;13:757–77.
https://doi.org/10.2147/TCRM.S117321
Wang C-L, Wu VC-C, Lee CH, Wu C-L, Chen H-M, Huang Y-T, et al. Incidences, risk factors, and clinical correlates of severe QT prolongation after the use of quetiapine or haloperidol. Heart Rhythm. 2024;21(3):321–8.
https://doi.org/10.1016/j.hrthm.2023.10.027
Erkan O, Ozturk N, Ozdemir S. Impact of quetiapine on ion channels and contractile dynamics in rat ventricular myocyte. European Journal of Pharmacology. 2024;976:176674.
https://doi.org/10.1016/j.ejphar.2024.176674
Zhuang W, Mun SY, Park M, Jeong J, Kim HR, Park H, et al. Second‐generation antipsychotic quetiapine blocks voltage‐dependent potassium channels in coronary arterial smooth muscle cells. Journal of Applied Toxicology. 2024;44(9):1446–53.
https://doi.org/10.1002/jat.4648
Lee HJ, Choi J-S, Choi BH, Hahn SJ. Effects of norquetiapine, the active metabolite of quetiapine, on cloned hERG potassium channels. Neuroscience Letters. 2018;664:66–73.
https://doi.org/10.1016/j.neulet.2017.11.029
Kim D-H, Park K-S, Park S-H, Hahn SJ, Choi J-S. Norquetiapine blocks the human cardiac sodium channel Nav1.5 in a state-dependent manner. European Journal of Pharmacology. 2020;885:173532.
https://doi.org/10.1016/j.ejphar.2020.173532
Stimmel GL, Gutierrez MA, Lee V. Ziprasidone: an atypical antipsychotic drug for the treatment of schizophrenia. Clinical Therapeutics. 2002;24(1):21–37.
https://doi.org/10.1016/S0149-2918(02)85003-2
Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT1A receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biological Psychiatry. 2000;48(3):229–37.
https://doi.org/10.1016/S0006-3223(00)00850-7
Greenberg WM, Citrome L. Ziprasidone for schizophrenia and bipolar disorder: a review of the clinical trials. CNS Drug Reviews. 2007;13(2):137–77.
https://doi.org/10.1111/j.1527-3458.2007.00008.x
Rosa AR, Franco C, Torrent C, Comes M, Cruz N, Horga G, et al. Ziprasidone in the treatment of affective disorders: a review. CNS Neuroscience & Therapeutics. 2008;14(4):278–86.
https://doi.org/10.1111/j.1755-5949.2008.00056.x
He L, Yu W, Song H, Li L, Shen Y, Zhang L, et al. Comparative risk of QTc prolongation induced by second-generation antipsychotics in the real world: retrospective cohort study based on a hospital information system. BJPsych Open. 2025;11(2):e42.
https://doi.org/10.1192/bjo.2024.871
Stollings JL, Boncyk CS, Birdrow CI, Chen W, Raman R, Gupta DK, et al. Antipsychotics and the QTc interval during delirium in the intensive care unit: a secondary analysis of a randomized clinical trial. JAMA Network Open. 2024;7(1):e2352034-e.
https://doi.org/10.1001/jamanetworkopen.2023.52034
Burton S, Heslop K, Harrison K, Barnes M. Ziprasidone overdose. American Journal of Psychiatry. 2000;157(5):835.
https://doi.org/10.1176/appi.ajp.157.5.835
House M. Overdose of ziprasidone. American Journal of Psychiatry. 2002;159(6):1061-a-2.
https://doi.org/10.1176/appi.ajp.159.6.1061-a
Manini AF, Raspberry D, Hoffman RS, Nelson LS. QT prolongation and torsades de pointes following overdose of ziprasidone and amantadine. Journal of Medical Toxicology. 2007;3(4):178.
https://doi.org/10.1007/BF03160936
Roe CM, Odell KW, Henderson RR. Concomitant use of antipsychotics and drugs that may prolong the QT interval. Journal of Clinical Psychopharmacology. 2003;23(2):197–200.
https://doi.org/10.1097/00004714-200304000-00013
Ducroq J, Printemps R, Le Grand M. Additive effects of ziprasidone and d,l-sotalol on the action potential in rabbit Purkinje fibres and on the hERG potassium current. Journal of Pharmacological and Toxicological Methods. 2005;52(1):115–22.
https://doi.org/10.1016/j.vascn.2005.04.001
Wu L, Shryock JC, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. The Journal of Pharmacology and Experimental Therapeutics. 2006;316(2):718–26.
https://doi.org/10.1124/jpet.105.094862
Kongsamut S, Kang J, Chen X-L, Roehr J, Rampe D. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. European Journal of Pharmacology. 2002;450(1):37–41.
https://doi.org/10.1016/S0014-2999(02)02074-5
Lacerda A, Kramer J, Shen K-Z, Thomas D, Brown A. Comparison of block among cloned cardiac potassium channels by non-antiarrhythmic drugs. European Heart Journal Supplements. 2001;3(Suppl K):K23–K30.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Orhan Erkan, Ayse Suna Dai, Nihal Ozturk, Semir Ozdemir

This work is licensed under a Creative Commons Attribution 4.0 International License.









